Examine the Henrys law constants in Table 14.4. Why is the constant for ammonia larger than the
Question:
Examine the Henry’s law constants in Table 14.4. Why is the constant for ammonia larger than the others?
(a) Because ammonia is polar and the other substances in the table are nonpolar.
(b) Because ammonia has the highest molar mass of the substances listed in the table.
(c) Because ammonia is nonpolar and the other substances in the table are polar.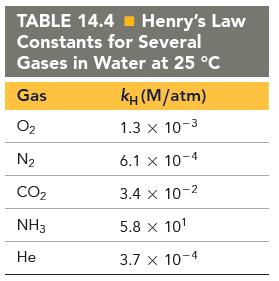
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





