Alloys of tin and lead are common solders. A solder is a metal alloy that melts at
Question:
Alloys of tin and lead are common solders. A solder is a metal alloy that melts at a relatively low temperature and connects two or more metal pieces. The technique of soldering is commonly used by plumbers to fuse copper water pipes and by electricians to affix electrical components to printed circuit boards. The table that follows lists tensile strength and hardness data for pure tin, pure lead, and two tin–lead alloys. Tensile strength is the maximum stress a stretched material can withstand before breaking. Hardness on the Brinell Scale is a measure of a substance’s resistance to indentation when subjected to impact by a steel ball (the higher the number, the greater the hardness).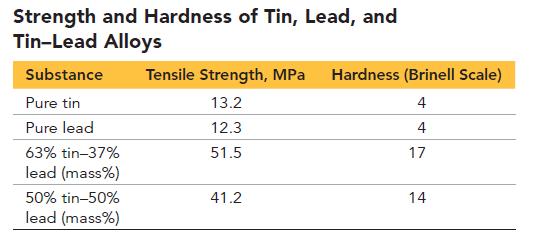
The tin–lead phase diagram in the graph that follows contains a eutectic point—a point on the phase diagram representing the lowest melting point. The eutectic composition has a sharp melting transition. All other compositions of tin–lead melt over a range of temperatures, beginning at the solidus line (the line separating the solid phase and the liquid–solid region) and ending at the liquidus line (the line separating the liquid phase and the liquid–solid region). Between these lines, a mixture of liquid and solid exists. This mixture is described as “pasty” because it can be worked into an optimal shape around the connection point of the two base metal pieces.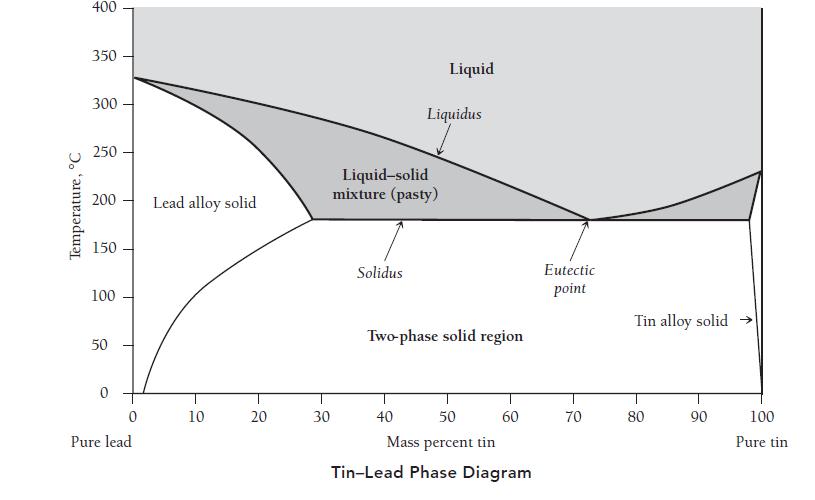
Use the information provided to answer the following questions:
a. Based on the data provided in the table, explain why tin–lead alloys are better solders than pure tin or pure lead.
b. Based on the phase diagram shown, do tin and lead form a miscible solid solution at all compositions?
c. According to the phase diagram, what are the melting points of pure tin and pure lead?
d. What composition of tin and lead constitutes a eutectic mixture, and what is the melting point of the eutectic mixture?
e. Why is a 63% tin/37% lead (mass %) alloy commonly used for electrical soldering projects?
f. What is the melting range of a 50% tin/50% lead (mass %) alloy?
Step by Step Answer:






