At 400 K, oxalic acid decomposes according to the reaction: H 2 C 2 O 4 (g)
Question:
At 400 K, oxalic acid decomposes according to the reaction:
H2C2O4(g) → CO2(g) + HCOOH(g)
In three separate experiments, the initial pressure of oxalic acid and final total pressure after 20,000 s are measured.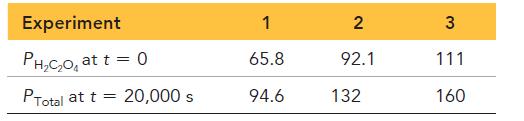
Find the rate law of the reaction and its rate constant.
Transcribed Image Text:
Experiment PH₂C₂O₂ at t = 0 PTotal at t = 20,000 s 1 65.8 94.6 2 92.1 132 3 111 160
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To find the rate law and rate constant of the reaction we can use the following steps Write the diff...View the full answer

Answered By

Lamya S
Highly creative, resourceful and dedicated High School Teacher with a good fluency in English (IELTS- 7.5 band scorer) and an excellent record of successful classroom presentations.
I have more than 2 years experience in tutoring students especially by using my note making strategies.
Especially adept at teaching methods of business functions and management through a positive, and flexible teaching style with the willingness to work beyond the call of duty.
Committed to ongoing professional development and spreading the knowledge within myself to the blooming ones to make them fly with a colorful wing of future.
I do always believe that more than being a teacher who teaches students subjects,...i rather want to be a teacher who wants to teach students how to love learning..
Subjects i handle :
Business studies
Management studies
Operations Management
Organisational Behaviour
Change Management
Research Methodology
Strategy Management
Economics
Human Resource Management
Performance Management
Training
International Business
Business Ethics
Business Communication
Things you can expect from me :
- A clear cut answer
- A detailed conceptual way of explanation
- Simplified answer form of complex topics
- Diagrams and examples filled answers
4.90+
46+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
At 500 K in the presence of a copper surface, ethanol decomposes according to the equation C2H5OH(g) CH3CHO(g) + H2(g) The pressure of C2H5OH was measured as a function of time, and the following...
-
There are two significant pieces of legislation that impact maritime security in the United States. These include the Maritime Transportation Security Act 2002 and the S.A.F.E. Port Act of 2006....
-
Visit www.guidestar.org and obtain the Form 990 for a local not-for-profit organization. a. Examine Part VIII of the 990 to determine gross receipts of the organization. b. Examine Part IX of the...
-
Why are the accounting requirements for job-order costing more demanding than those for process costing?
-
Given the representation: Can you determine the g(-) if the M T (X T ) is the payoff of an plain vanilla European call option at expiration? That is, if M T (X T ) is given by: M T (X T ) = max [X T?...
-
Refer to information in QS 21-14. Compute the overhead volume variance for November and classify it as favorable or unfavorable. Data From QS 21-14 AirPro Corp. reports the following for November....
-
Kelly Jones and Tami Crawford borrowed $15,000 on a 7-month, 8% note from Gem State Bank to open their business, JC's Coffee House. The money was borrowed on June 1, 2017, and the note matures...
-
My elevator pitch for the purchase of an MRI machine is as follows: Hi there, my name is Kayla and I am one of the administrators here at Highlands Regional. Did you know that our MRI machine was...
-
Dinitrogen pentoxide decomposes in the gas phase to form nitrogen dioxide and oxygen gas. The reaction is first order in dinitrogen pentoxide and has a half-life of 2.81 h at 25 C. If a 1.5-L...
-
At 700 K, acetaldehyde decomposes in the gas phase to methane and carbon monoxide. The reaction is: CH 3 CHO(g)CH 4 (g) + CO(g) A sample of CH 3 CHO is heated to 700 K, and the pressure is measured...
-
E-learning is very popular today. What specific advice would you give anyone launching a corporate e-learning program who wants to make it as effective as possible? Do you think e-learning can ever...
-
Summarize the position that a dividend policy may be irrelevant with regard to the firms stock price.
-
What is the basic controversy surrounding capital structure theory?
-
What is the typical frequency with which cash dividends are paid to investors?
-
Distinguish among the (a) declaration date, (b) date of record, (c) ex-dividend date.
-
Explain the different types of value.
-
Furniture costing $ 55,000 is sold at its book value in 2013. Acquisitions of furniture total $ 45,000 cash, on which no depreciation is necessary because it is acquired at year- end. What is the...
-
Refer to the data in QS 10-1. Based on financial considerations alone, should Helix accept this order at the special price? Explain.
-
A beam of protons, at first moving slowly, is accelerated to higher and higher speeds. When the protons are moving slowly, the beam spreads out, but when they are moving fast, the beam diameter...
-
ZA current is passed through a helical (corkscrew-shaped) spring. What, if anything, do you think happens to the length of the spring?
-
A physicist is equipped to measure electric, magnetic, and gravitational fields. Which will she detect when a proton moves past her? When she moves past a proton?
-
There are 1,700 stocks in the Value Line index. How many covariances would have to be computed to use the Markowitz full-covariance model? Use the data in Table for Problems Security Covariances A B...
-
1. Briefly explain the computerised product cycle in the manufacturing environment. 2. What are the functions that get benefited by the use of computers in design and manufacturing functions? 3....
-
find 4) y sec(x + 1) 5) y = Incos x 1 6) y=ln xx+1

Study smarter with the SolutionInn App


