At 700 K, acetaldehyde decomposes in the gas phase to methane and carbon monoxide. The reaction is:
Question:
At 700 K, acetaldehyde decomposes in the gas phase to methane and carbon monoxide. The reaction is: CH3CHO(g)¡CH4(g) + CO(g)
A sample of CH3CHO is heated to 700 K, and the pressure is measured as 0.22 atm before any reaction takes place. The kinetics of the reaction are followed by measurements of total pressure, and these data are obtained: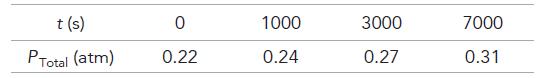
Find the rate law, the rate constant, and the total pressure after 2.00 * 104 s.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





