Balance the redox reactions by following the steps in the text. Rotate through the group, having each
Question:
Balance the redox reactions by following the steps in the text.
Rotate through the group, having each group member do the next step in the process and explain that step to the rest of the group.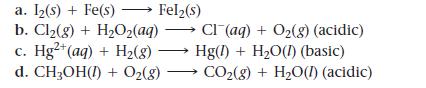
Transcribed Image Text:
a. Iz(s) + Fe(s) > Fel₂(s) b. Cl₂(g) + H₂O₂(aq) c. Hg²+ (aq) + H₂(8) d. CH3OH(1) + O₂(g) Cl(aq) + O₂(g) (acidic) Hg(1) + H₂O(1) (basic) CO₂(g) + H₂O(1) (acidic)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
a 12s Fes Fels 1 Assign Oxidation States Fe is oxidized and I has an oxidation state of 1 2 Write HalfReactions Oxidation HalfReaction 12s 12e Fe3 Red...View the full answer

Answered By

Wahome Michael
I am a CPA finalist and a graduate in Bachelor of commerce. I am a full time writer with 4 years experience in academic writing (essays, Thesis, dissertation and research). I am also a full time writer which assures you of my quality, deep knowledge of your task requirement and timeliness. Assign me your task and you shall have the best.
Thanks in advance
4.90+
63+ Reviews
132+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
THIRD AVENUE SOFTWARE HEALTH-CARE APP PROJECT This case is new for the ninth edition of Information Technology Project Management . The case provides an opportunity to apply agile and Scrum...
-
If a natural disaster, such as the 2010 drought in Russia, hits food production, use supply and demand analysis to figure out how this affects consumers and producers. Does everyone lose or are some...
-
Eggars produced 572,000 units last year. The information on the actual costs and budgeted costs at actual production of four activities follows. Required: Prepare an activity-based performance report...
-
Explain the difference between crowding out and crowding in. Given the current state of the economy, which effect would you expect to dominate today?
-
A contract is created to refurbish a luxury yacht: new color schemes, new furniture, new wall and floor coverings, new light fixtures, and window treatmentsthe whole works. Of course, it is not just...
-
In December 2011, the Hamilton County Board of Commissioners established the Hamilton County OPEB Trust Fund. Retired employees of Hamilton County can participate in post-employment benefits through...
-
The N excess/deficit factor for 100 kg of an organic material that contained 60% C (carbon) and 0.5% N (nitrogen) would likely be (assuming that 35% of the carbon is metabolized by microorganisms and...
-
In this chapter, you have seen that the voltage of an electrochemical cell is sensitive to the concentrations of the reactants and products in the cell. As a result, electrochemical cells can be used...
-
Which oxidizing agent will oxidize Br but not Cl? a. K 2 Cr 2 O 7 (in acid) b. KMnO 4 (in acid) c. HNO 3
-
A hydrocarbon of unknown structure has the formula C8H10. On catalytic hydrogenation over the Lindlar catalyst, 1 equivalent of H2 is absorbed. On hydrogenation over a palladium catalyst, 3...
-
Mars Inc. is considering the purchase of a new machine that costs $80,000. This machine will reduce manufacturing costs by $20,000 annually. Mars will use the 3- year MACRS method (shown below) to...
-
Differentiate f(x)=2ln(2x+5) - tan-(2x) +
-
Owing to his wife's ill-health during the COVID-19 pandemic, Paul sold a gift shop and an investment property in NSW and then moved to Queensland for retirement on 30 June 2022. Paul acquired the...
-
The shoe brand, Nike, faced a marketing crisis in 2018 when the brand published its Dream Crazy ad on September 5, 2018, with Colin Kaepernick as the narrator. What are the direct and digital...
-
Why did the Union prevail, and the Confederacy lose, the Civil War?
-
Would a project manager ever consider crashing a noncritical activity in a project network? Explain convincingly.
-
Michelles trust is subject to 3.8% surtax on the lesser of the trusts net investment income or the excess of the trusts adjusted gross income over the $12,400 threshold (the highest trust tax rate)....
-
With sufficient energy, it?s possible to eject an electron from an inner atomic orbital. A higher-energy electron will then drop into the unoccupied state, emitting a photon with energy equal to the...
-
With sufficient energy, it?s possible to eject an electron from an inner atomic orbital. A higher-energy electron will then drop into the unoccupied state, emitting a photon with energy equal to the...
-
With sufficient energy, it?s possible to eject an electron from an inner atomic orbital. A higher-energy electron will then drop into the unoccupied state, emitting a photon with energy equal to the...
-
A laser beam of wavelength 632 nm is incident from air into water at an angle of 33 degrees from the perpendicular to the surface of the water. What is the angle that the laser beam is refracted?
-
Find the missing side lengths. Leave your answers as radicals in simplest form. 1) b 45 22 a Click Save and Submit to save and submit. Click Save All Answers to save all answers 2) 4 X 45 y
-
Multiply and simply the resulting expression with positi (-10x-5y-2). (-9x4y) Please show all of the steps for your solution. Multiply and simply the resulting expression with positive

Study smarter with the SolutionInn App


