Calculate E cell for each balanced redox reaction and determine if the reaction is spontaneous as written.
Question:
Calculate E°cell for each balanced redox reaction and determine if the reaction is spontaneous as written.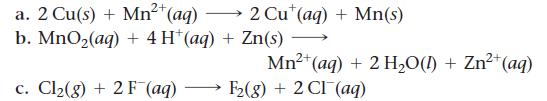
Transcribed Image Text:
2+ a. 2 Cu(s) + Mn²+ (aq) b. MnO₂(aq) + 4H+ (aq) + Zn(s) c. Cl₂(g) + 2 F (aq) 2 Cut (aq) + Mn(s) 2+ Mn²+ (aq) + 2 H₂O(1) + Zn²+ (aq) F₂(g) + 2 CI (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 170 V nonsp...View the full answer

Answered By

Shehar bano
I have collective experience of more than 7 years in education. my area of specialization includes economics, business, marketing and accounting. During my study period I remained engaged with a business school as a visiting faculty member and did a lot of business research. I am also tutoring and mentoring number of international students and professionals online for the last 7 years.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate E cell for each balanced redox reaction and determine if the reaction is spontaneous as written. a. O(g) + 2 HO(1) + 4 Ag(s) b. Br(1) 21 (aq) c. PbO (s) + 4H+ (aq) + Sn(s) 4 OH(aq) + 4 Ag+...
-
Toplob Inc. ("Toplob") provides employment consulting services. These services range from maintaining payroll records to taxation services, as well as general business advisory and consulting to new...
-
Which 3 accounts can't be merged in the chart of accounts?
-
At the beginning of last year, Diekow Productions set variable overhead standards of 10 machine hours at a rate of $ 10 per hour for each product produced. During the year, 10,800 machine hours were...
-
Give examples of U.S. banks facing different risks in international lending.
-
In 1988, the Upper Deck Company was a company with an idea for a better baseball card: one that had a hologram on it. By the 1990s, the firm was a major corporation worth at least a quarter of a...
-
Logan Krause started her own consulting firm, Krause Consulting, on May 1, 2017. The trial balance at May 31 is as follows. In addition to those accounts listed on the trial balance, the chart of...
-
Oil having a density of 921 kg/m floats on water. A rectangular block of wood 4.41 cm high and with a density of 963 kg/m floats partly in the oil and partly in the water. The oil completely covers...
-
Determine whether or not each metal dissolves in 1 M HIO 3 . For those metals that do dissolve, write a balanced redox equation for the reaction that occurs. a. Au b. Cr
-
Determine whether or not each metal dissolves in 1 M HNO 3 . For those metals that do dissolve, write a balanced redox reaction showing what happens when the metal dissolves. a. Cu b. Au
-
Burchards Ethical Dissonance Cycle Model suggests this was a failure of which of the following? a. Low Organization Ethics, Low Individual Ethics Low Low b. High Organizational Ethics, Low Individual...
-
Non-linearity is one important property that makes DES secure. This problem verifies the nonlinear property by computing the output of S for several pairs of inputs. Show that: S(x1) S(x2) S(x1 x2)...
-
Treasury secretary Hank Paulson and the President of the NY Fed Timothy F. Geithner were involved in the discussions of the deal. a) Assume that the only goal that the NY Fed and the Treasury pursued...
-
Explain why the Uruguay round of negotiations launched in September 1986 is held to be the most successful of the 8 General Agreement on Tariffs and Trade rounds of negotiations.
-
Oak Bay Software has 10.5% coupon bonds on the market with 18 years to maturity. The bonds make semiannual payments and currently sell for 108.1% of par. What is the YTM? (Do not round intermediate...
-
Find an equation of the plane passing through the given point and parallel to the given plane. a. (2, -4,5), z = 2x + 3y b. (0,6,-4), 2x - 3y - 6z = 0 10. Find an equation of the plane passing...
-
List three issues and environmental forces facing human resource management in the health care setting today. Cite their impact and identify at least two current initiatives being used to overcome...
-
The age-old saying for investing is "buy low and sell high," but this is easier said than done. Investors who panic about falling prices sell their investments, which in turn lowers the price and...
-
Imagine that we have Youngs Experiment, where one of the two pinholes is now covered by a neutral-density filter that cuts the irradiance by a factor of 10, and the other hole is covered by a...
-
Show (for normally incident plane waves) that if an aperture has a center of symmetry (i.e., if the aperture function is even), then the diffracted field in the Fraunhofer case also possesses a...
-
Suppose a given aperture produces a Fraunhofer field pattern E(Y, Z). Show that if the apertures dimensions are altered such that the aperture function goes from A(y, z) to A(αy,...
-
3. Match each function with its graph. a) f(x)=4(2) b) y=2log.x c) y=-4lnx d) f(x)=4 i) iii) ii) -2-1 7 6- 5 4 3 2- T 4 121 2 3 4 14 1- -2- -2- 3- 4- 3 4 iv) -2 2- 1 -5 + 324 4 3- 2 4 2 3. 4 -1- -2 3...
-
DU recently paid a dividend of $1.60. An analyst expects that the firm's dividend rate will grow at a constant rate of 2% indefinitely. He also determines DU's beta is 1.4, the risk-free rate is...
-
Reliable Motors shares are expected to pay dividends of $2.00, $1.15, and $1.20 at the end of each of the next three years, respectively. The investor expects the price of the shares at the end of...

Study smarter with the SolutionInn App


