Calculate H rxn for the combustion of octane (C 8 H 18 ), a component of gasoline,
Question:
Calculate ΔHrxn for the combustion of octane (C8H18), a component of gasoline, by using average bond energies and then calculate it using enthalpies of formation from Appendix IIB.
What is the percent difference between your results? Which result would you expect to be more accurate?
Appendix IIB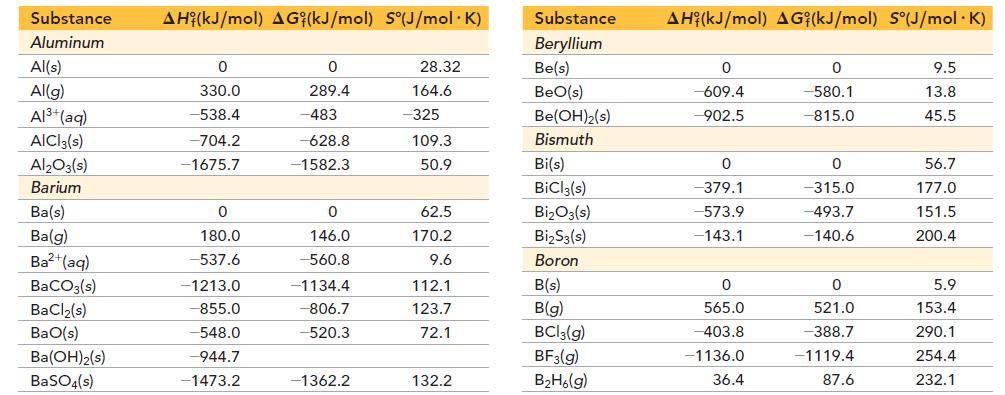
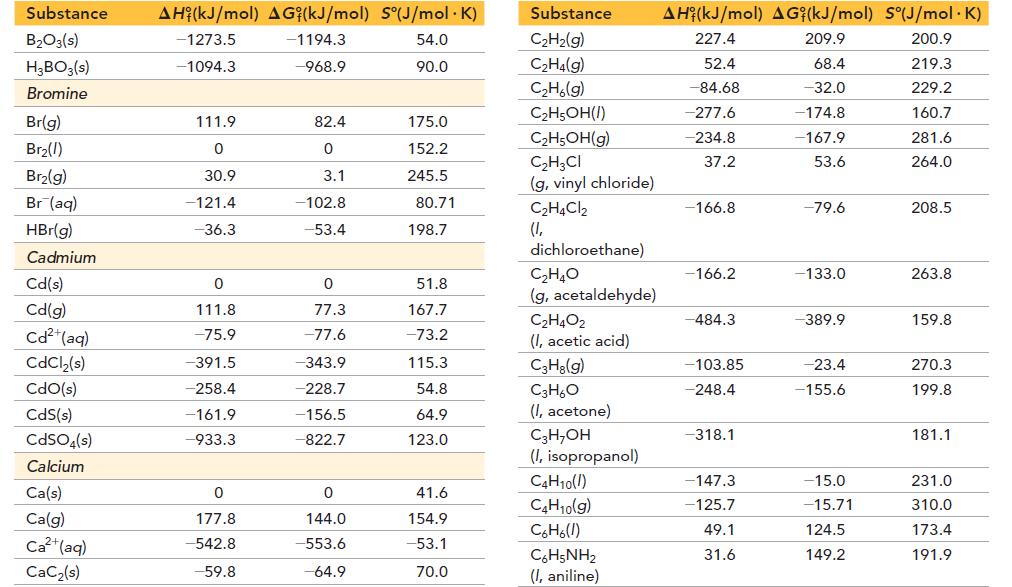
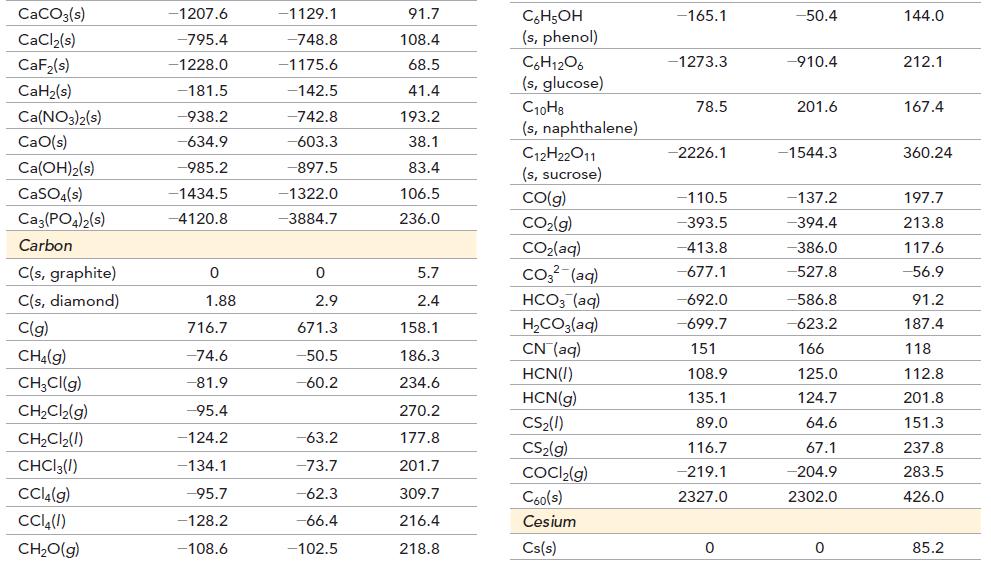
Transcribed Image Text:
Substance Aluminum Al(s) Al(g) Al³+(aq) AlCl3(s) Al₂O3(s) Barium Ba(s) Ba(g) Ba²+(aq) BaCO3(s) BaCl₂(s) BaO(s) Ba(OH)₂(s) BaSO4(s) AH (kJ/mol) AG (kJ/mol) S°(J/mol. K) 0 330.0 -538.4 -704.2 -1675.7 0 180.0 -537.6 -1213.0 -855.0 -548.0 -944.7 -1473.2 0 289.4 -483 -628.8 -1582.3 0 146.0 -560.8 -1134.4 -806.7 520.3 -1362.2 28.32 164.6 -325 109.3 50.9 62.5 170.2 9.6 112.1 123.7 72.1 132.2 Substance Beryllium Be(s) BeO(s) Be (OH)2(s) Bismuth Bi(s) BiCl3(s) Bi₂O3(s) Bi₂S3(s) Boron B(s) B(g) BCI 3(g) BF3(g) B₂H6(g) AH (kJ/mol) AG (kJ/mol) S°(J/mol. K) 0 -609.4 -902.5 0 -379.1 -573.9 -143.1 0 565.0 -403.8 -1136.0 36.4 0 -580.1 -815.0 0 -315.0 -493.7 -140.6 0 521.0 -388.7 -1119.4 87.6 9.5 13.8 45.5 56.7 177.0 151.5 200.4 5.9 153.4 290.1 254.4 232.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Average bond energies and enthalpies of formation are the two approaches we can use to compute Hrxn ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate ? H rxn for the combustion of octane (C 8 H 18 ), a component of gasoline, by using average bond energies. Calculate ? H rxn for the combustion of octane by using enthalpies of formation...
-
A city of 100,000 people uses approximately 1.0 * 10 11 kJ of energy per day. Suppose all of that energy comes from the combustion of liquid octane (C 8 H 18 ) to form gaseous water and gaseous...
-
It is desired to control the amount of CO in the products of combustion of octane C8H18 so that the volume fraction of CO in the products is less than 0.1 percent. Determine the percent theoretical...
-
Be able to explain how land policy shaped economic growth in the United States.
-
The BSN Company would like a hard copy report of all the current vendors in its database. Figure provides a suggested format for the report. Note that your report header should include the company...
-
The contribution format income statement for Huerra Company for last year is given below: The company had average operating assets of $2,000,000 during the year. Required: 1. Compute the companys...
-
On February 20, 2009, Cedar Valley Aviation, a wholly owned subsidiary of Aerial Services, Inc. (ASI), brought a Piper 522AS (Cheyenne II) in for maintenance to Des Moines Flying Service, Inc....
-
Nguyen Company has the following stock outstanding: Common Stock Preferred Stock 60,000 shares ......5,000 shares $1 par value .......$60 par, $3 dividend The amount available for dividends this year...
-
Simplify: 6/8p 98p.
-
Draw the Lewis structure for urea, H 2 NCONH 2 , one of the compounds responsible for the smell of urine. (The central carbon atom is bonded to both nitrogen atoms and to the oxygen atom.) Does urea...
-
Draw the Lewis structure for HCSNH 2 . (The carbon and nitrogen atoms are bonded together, and the sulfur atom is bonded to the carbon atom.) Label each bond in the molecule as polar or nonpolar.
-
Refer to the Acoustical Science & Technology (Vol. 35, 2014) study of irrelevant speech effects, Exercise 8.47 (p. 392). Recall that 71 subjects performed a memorization task under two conditions:...
-
A European apparel manufacturer has production facilities in Italy and China to serve its European market, where annual demand is for 1.9 million units. Demand is expected to stay at the same level...
-
Choose the optimal lot size given fixed ordering costs in a supply chain.
-
A clothing manufacturer is setting up capacity in Spain and Poland for the next three years. Annual demand in each market is 1.5 million units and is likely to remain at that level. The two choices...
-
________ is a service factor performance characteristic of manufacturer or distributor storage with customer pickup that is lower than other options because of the lack of home delivery. Experience...
-
Consider an auto parts retailer implementing distributor storage with last-mile delivery. List some advantages and disadvantages that the retailer would experience with this method.
-
On October 1, Bandor Company sold land (that cost $30,000) on credit for $35,000. The buyer issued an 8%, 12-month note for this amount, with the interest to be paid on the maturity date. Prepare...
-
Write electron configurations for the following ions, and determine which have noble-gas configurations: (a) Cd2+ (b) p3- (c) Zr4+ (d) Ru3+ (e) As3- (f) Ag+
-
Consider the motion of a marble as it falls to the bottom of a jar of honey. Experiments show (see also Chapter 3) that the marble moves with a constant velocity. Applying Newtons first law, does...
-
Figure P2.17 shows several hypothetical velocitytime graphs. For each case, sketch qualitatively the corresponding accelerationtime graph. Figure P2.17 Case 2 Case 1 Case 3
-
Consider the motion of the Moon as it orbits the Earth. (a) Is the Moons acceleration zero or nonzero? Explain. (b) If the Moon has a nonzero acceleration, what force is responsible?
-
Discuss the benefits that your company would receive from nurturing, enabling and empowering its talent
-
Define and describe the pathophysiology and risk factors of Type One Diabetes mellitus.
-
Develop profiles of target customers Find out who and where are primary customers are. Create profiles of the main customer types and design a marketing plan and sales campaign to attract them as...
Cow Talk The Environment In Modern North America Volume 8 1st Edition - ISBN: 0806191910 - Free Book

Study smarter with the SolutionInn App


