Calculate H rxn for the reaction: Use the following reactions and given Hs: CaO(s) + CO2(g) CaCO3(s)
Question:
Calculate ΔHrxn for the reaction:![]()
Use the following reactions and given ΔH’s: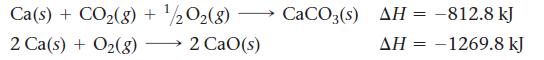
Transcribed Image Text:
CaO(s) + CO2(g) CaCO3(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate Hrxn for the reaction shown in the image we can use the followi...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A builder gets a construction loan of 10 million. For simplicity, assume the loan has an interest rate at j1=6%. The loan does not require payment during the construction process. Construction will...
-
Calculate S values for the following reactions by using tabulated values from Appendix C. In each case explain the sign of S. (c) HNO3(g) NH3 (g)- NH4NO3(s) 2 Fe203(s)4 Fe(s) 302(g) CaCO3(s,calcite)...
-
Stenback Exercise Equipment, Inc. reported the following financial statements for 2016: Requirements 1. Compute the amount of Stenback Exercises acquisition of plant assets. Assume the acquisition...
-
At April 30 the bank reconciliation of Guardado Company shows three outstanding checks: No. 254 $650, No. 255 $700 and No. 257 $410. The May bank statement and the May cash payments journal are given...
-
Compute the Fermi energy of potassium by making the simple approximation that each atom contributes one free electron. The density of potassium is 851 kg/m', and the mass of a single potassium atom...
-
After the positrons were annihilated, the energy density of the universe was dominated by the photons and the neutrinos. Show that the energy density in that era was given by \(u_{\text {total...
-
Houston-based Advanced Electronics manufactures audio speakers for desktop computers. The following data relate to the period just ended when the company produced and sold 42,000 speaker sets: Sales...
-
What are some business benefits of adopting the relational database model within an organization? How does a relational model create or enhance business value? Are there any limitations to using a...
-
Calculate H rxn for the reaction: Use the following reactions and given Hs: CH4(g) + 4 Cl2(g) CC14(g) + 4 HCl(g)
-
Calculate H rxn for the reaction: Use the following reactions and given Hs: FeO3(s) + 3 CO(g) 2 Fe(s) + 3 CO2(8)
-
In one school district there are 17,000 voters choosing among three alternative pro-posed levels of spending $3,000 per student, $6,000 per student, and $10,000 per student. The preferences of the...
-
Forensic Psychology's Role in the Legal SystemResources PSYC Discussion Participation Scoring Guide . In this unit, you were introduced to forensic psychology as a subfield of psychology as well as...
-
Why is proper inventory valuation so important? Why does an understated ending inventory understate net income for the period by the same amount? Why does an error in ending inventory affect two...
-
Do liquids dissolve structures blocking their path, or do they merely circumnavigate them? Discuss the impact of increased liquidity and gaseousness on hierarchical social structures. Is the current...
-
Decision making is management"s most important function. Do you agree? Why or why not?
-
During the first quarter of the year, Swifty Corporation generated sales revenue of $1375000 on sales of 55000 units of its wireless earbuds. The break-even point is 22000 units. What is the...
-
At what amount does Apple report cash and cash equivalents in its 2011 consolidated balance sheet?
-
Write the binomial probability in words. Then, use a continuity correction to convert the binomial probability to a normal distribution probability. P(x 110)
-
Singlet carbenes add to alkenes to yield cyclopropanes. Stereochemistry is maintained, meaning that cis- and trans-substituted alkenes give cis- and trans-substituted cyclopropanes, respectively; for...
-
Some symmetry operations can be carried out physically using a ball-and-stick model of a molecule without disassembly and reassembly and others can only be imagined. Give two examples of each...
-
Predict the number of chemically shifted 1 H peaks and the multiplet splitting of each peak that you would observe for 1,1,1,2-tetrachloroethane. Justify your answer. I H1 --c-CI CI | Cl
-
Marisa is 40 years old has several 401(k) plans from moving between employers. She's getting overwhelmed by all the plans, and her friend tells her she can roll them over into an IRA. Marisa decides...
-
What is the main advantage of an unconditional policy commitment? OA. It provides a significant amount of certainty, which makes it easier for markets and households to make decisions about the...
-
When you freeze the top row of a worksheet, what part of the worksheet can you scroll?

Study smarter with the SolutionInn App


