Calculate the change in Gibbs free energy for each of the sets of H rx n ,
Question:
Calculate the change in Gibbs free energy for each of the sets of ΔHrxn, ΔSrxn, and T given in Problem 42. Predict whether or not each reaction is spontaneous at the temperature indicated.
Problem 42
Given the values of ΔHrxn, and T, determine ΔSrxn, and predict whether or not each reaction is spontaneous.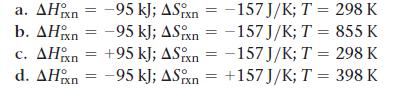
Transcribed Image Text:
a. AH n = -95 kJ; ASxn-157J/K; T = 298 K b. AH = -95 kJ; ASn = -157 J/K; T = 855 K c. AHxn = -157 J/K; T = 298 K +95 kJ; ASn = -95 kJ; ASixn -95 kJ; ASn = d. AH = +157J/K; T = 398 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To calculate the change in Gibbs free energy G for each reaction we use the equation G H TS We are g...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the change in Gibbs free energy for each of the sets of H rx n , S rxn , and T given in Problem 41. Predict whether or not each reaction is spontaneous at the temperature indicated. Problem...
-
Using data from Appendix C, calculate the change in Gibbs free energy for each of the following reactions. In each case indicate whether the reaction is spontaneous at 298 K under standard...
-
There are 2 retail companies A and B with some financial information listed in the table below. One company is a supermarket, the other is a jewellery retailer. Distinguish which is company A and...
-
when taking a lead role for supplier selection can often help Multiple select question. establish supplier quality standards. determine price. develop the engineering specifications. set conditions...
-
What are the consequences of each stage of marketing development on the potential for industrial goods within a country? For consumer goods?
-
ITT is a conglomerate with divisions in a wide variety of businesses. Historically, top management of ITT has considered annual earnings growth to be a primary measure of corporate performance....
-
Increasingly, we are seeing email used in cases involving defendants located in foreign countries. Plaintiffs filed suit against four Defendants: Qingdao Sunflare New Energy Co., Skone Lighting Co.,...
-
Aubrey Inc. issued $4,000,000 of 10%, 10-year convertible bonds on June 1, 2014, at 98 plus accrued interest. The bonds were dated April 1, 2014, with interest payable April 1 and October 1. Bond...
-
In return for an investment of $34318 in a fixed interest security, you will receive $134 at the end of each half year plus your money back on redemption in 12 years. You intend to deposit all the...
-
Calculate the free energy change for this reaction at 25 C. Is the reaction spontaneous? C3H8(g) + 5O(g) 5 O(g) 3 CO(g) + 4HO(g) AHin = -2217 kJ; ASixn 101.1 J/K rxn
-
Given the values of H rxn , and T, determine S rxn , and predict whether or not each reaction is spontaneous. a. AH n = -95 kJ; ASxn-157J/K; T = 298 K b. AH = -95 kJ; ASn = -157 J/K; T = 855 K c....
-
An AM modulator has output x c (t) = 40 cos [20(200)t] + 5 cos[2(180)t] + 5 cos[2(220)t] Determine the modulation index and the efficiency.
-
What are strategies adjustment messages that salvage customers' trust and promote further business?
-
What might separate the clients we work with from those that do not require support?
-
Describe strategies using examples of children's literature that motivate interest in reading and writing. How would these strategies increase a child's vocabulary? Why is storytelling important? How...
-
Address the human resource implications for a company as it goes international as well as your role in HR in this growth. 1. What current HRM practices that will need to be modified as the...
-
Why does Nestle want to list Alcon? What is an ADR? What advantages does it offer relative to a domestic listing? Why do firms cross-list? What are the consequences of cross-listing? How do they...
-
Assume that the privately held technology company decides to become a publicly traded company on the NASDAQ and is required to adopt International Financial Reporting Standards (IFRS). Determine the...
-
Hardin Services Co. experienced the following events in 2016: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
A long, non-uniform board of length 8.0 m and mass m = 12 kg is suspended by two ropes as shown in Figure P8.83. If the tensions in the ropes are mg/3 (on the left) and 2mg/3 (on the right), what is...
-
Consider again the problem of a tipping car in Example 8.6. This time, instead of applying a force F to the car, assume the car is traveling around a curve on a level road. Let the radius of...
-
A solid wood ball is rotating about an axis that passes through its center. If its angular speed is doubled, (a) by what factor does its rotational kinetic energy change? (b) By what factor does its...
-
The expected returns for Acme Company are shown below: Economy Probability Return Boom 0.10 20% Above average 0.20 15% Average 0.40 10% Below average 0.20 5% Recession 0.10 -10% Calculate expected...
-
Bayesian Classifiers: Shyam is a CS 4375 student. Recently, his mood has been highly influenced by three factors: the weather (W), his study habits (S), and whether his neighbor is at home or not...
-
17. Parents Jacob and Emma have had two children, Samuel and Matthew. Baby Samuel died at the age of nine days. When baby Matthew has trouble feeding, his parents take him in to the doctor. He is...

Study smarter with the SolutionInn App


