Calculate the change in Gibbs free energy for each of the sets of H rx n ,
Question:
Calculate the change in Gibbs free energy for each of the sets of ΔHrxn, ΔSrxn, and T given in Problem 41. Predict whether or not each reaction is spontaneous at the temperature indicated.
Problem 41
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous.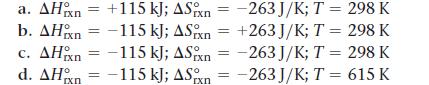
Transcribed Image Text:
a. AH n = +115 kJ; ASxn=-263 J/K; T = 298 K b. AH n = -115 kJ; ASn = +263 J/K; T = 298 K c. AH n = -115 kJ; ASn=-263 J/K; T = 298 K d. AH = -115 kJ; ASn=-263 J/K; T = 615 K ΙΠ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a 193 10 5 J nonspont...View the full answer

Answered By

Emily Grace
With over a decade of experience providing top-notch study assistance to students globally, I am dedicated to ensuring their academic success. My passion is to deliver original, high-quality assignments with fast turnaround times, always striving to exceed their expectations.
4.90+
3+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the change in Gibbs free energy for each of the sets of H rx n , S rx n , and T given in Problem 42. Predict whether or not each reaction is spontaneous at the temperature indicated....
-
Using data from Appendix C, calculate the change in Gibbs free energy for each of the following reactions. In each case indicate whether the reaction is spontaneous at 298 K under standard...
-
There are 2 retail companies A and B with some financial information listed in the table below. One company is a supermarket, the other is a jewellery retailer. Distinguish which is company A and...
-
You have $300,000 saved for retirement. Your account earns 6% interest. How much will you be able to pull out each month, if you want to be able to take withdrawals for 20 years? $ Get help: Video
-
Select a country in the agricultural and raw materials stage of economic development and discuss what changes will occur in marketing when it passes to a manufacturing stage.
-
Ross Clark, president of Clark Hotels, wishes to issue a press release to bolster his companys image and maybe even its stock price, which has been gradually falling. As controller, you have been...
-
The accounts and June 30, 2007, balances of Cromwell Company are arranged in no particular order: Requirements 1. Prepare the company's classified balance sheet in account format at June 30, 2007. 2....
-
If researchers know that consumers in various geographic regions respond quite differently to a product category, such as tomato sauce, is area sampling appropriate? Why or why not?
-
1. Construct a sales budget for FlashKick for the first three months of the coming year. Show total sales for each product line by month and in total for the first quarter. If required, round your...
-
Calculate the free energy change for this reaction at 25 C. Is the reaction spontaneous? C3H8(g) + 5O(g) 5 O(g) 3 CO(g) + 4HO(g) AHin = -2217 kJ; ASixn 101.1 J/K rxn
-
Given the values of H rxn , and T, determine S rxn , and predict whether or not each reaction is spontaneous. a. AH n = -95 kJ; ASxn-157J/K; T = 298 K b. AH = -95 kJ; ASn = -157 J/K; T = 855 K c....
-
Neighborhood Supermarkets is preparing to go public, and you are asked to assist the firm by preparing its statement of cash flows for 20X1. Neighborhoods balance sheets at December 31, 20X0, and...
-
An oriental rug is 5 feet longer than it is wide. If the diagonal of the rug is 12 feet, find its dimensions to the nearest tenth of a foot. width length ft
-
Background Alice Fabian is an entrepreneur from Belgium. She trades gourmet chocolate in the European Union. Alice arrived in Singapore on 27 January 2020 to assess market demand for her chocolates...
-
MedTech Ltd (MedTech) imports a number of pharmaceutical products. In order to hedge its foreign currency transactions, MedTech entered into a number of forward rate agreements this year. Prior to...
-
Hill describes the inclusion of the Project Office in the PMO Competency Continuum as an uncertain fit due to: Group of answer choices 1.The inclusion of the activities of more than one project...
-
Given a vehicle mass of 1400kg, wheel track of 1.2m, bump height of 0.2m and front to rear axle weight ratio of 60:40, determine the maximum torque generated by the bum height and overall torsional...
-
In 2017, Wainwright Company has net credit sales of $1,300,000 for the year. It had a beginning accounts receivable (net) balance of $101,000 and an ending accounts receivable (net) balance of...
-
Orange juice producers are dismayed and puzzled. An economist told them that the reason the demand for orange juice fell is that a new technology allow tomato producers to pick ripe tomatoes more...
-
Repeat Problem 10.55 for flow rates of 7.5 gal/min and 10.0 gal/min. Problem 10.55 For the data from Problem 10.53, compute the equivalent value of the resistance coefficient K if the pressure drop...
-
Repeat Problem 10.57 for flow rates of 7.5 gal/min and 10.0 gal/min. Problem 10.57 For the data from Problem 10.53, compute the flow coefficient C V as defined in section 10.13. The oil has a...
-
Compute the specific weight of natural gas at 4.50 inH 2 O and 55F.
-
Suppose a Summons and Complaint were filed on February 16, 2021 and personally served on the defendant on March 1, 2021, please calendar the following dates: a the day Proof of Service of Summons and...
-
In light of your personal experience, what strategies or approaches do you believe could be effective in creating a workplace environment where employees from diverse cultural backgrounds feel both...
-
1. Evaluate the performance management appeals process and its connection to the employee performance. very important no to give short answers very important to give a detailed explanation very...

Study smarter with the SolutionInn App


