Calculate the equilibrium constant for each of the reactions in Problem 65. Problem 65 Use tabulated electrode
Question:
Calculate the equilibrium constant for each of the reactions in Problem 65.
Problem 65
Use tabulated electrode potentials to calculate ΔG°rxn for each reaction at 25 °C.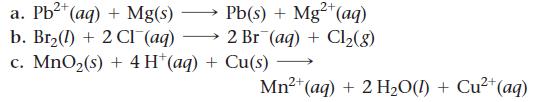
Transcribed Image Text:
a. Pb²+ (aq) + Mg(s) b. Br₂() + 2 CI (aq) c. MnO₂(s) + 4H+ (aq) + Cu(s) Pb(s) + Mg2+ (aq) 2 Br (aq) + Cl₂(8) 2+ Mn²+ (aq) + 2 H₂O(l) + Cu²+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
a 531 x ...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the equilibrium constant for each of the reactions in Problem 66. Problem 66 Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. 2+ a. 2 Fe+ (aq) + 3 Sn(s) 2...
-
Calculate the equilibrium constant for each of the reactions at25 ?C. Standard Electrode Potentials at 25 ?C Reduction Half-Reaction E ?(V) Pb2+( a q )+2 e ? ?Pb( s ) -0.13 Mg2+( a q )+2 e ? ?Mg( s )...
-
Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. 2+ a. 2 Fe+ (aq) + 3 Sn(s) 2 Fe(s) + 3 Sn+ (aq) b. O(g) + 2 HO(l) + 2 Cu(s) 4 OH(aq) + 2 Cu+ (aq) c. Br(1) + 21 (aq)...
-
1. What are some other ways in which HEALTHeLINK could be used to support public health activities?
-
Prefabricated houses are the specialty of Affordable Homes, Inc., of Corsicana, Texas. Although Affordable Homes produces many models, the companys best-selling model is the Welcome Home, a...
-
What variables affect the value of a call option?
-
This appeal involves the validity of a will executed in contravention of an earlier contract to make mutual wills. A husband and wife signed a contract to make mutual wills and then executed those...
-
The current sections of Nasreen Inc.s balance sheets at December 31, 2013 and 2014, are presented here. Nasreens net income for 2014 was $153,000. Depreciation expense was $24,000. Instructions...
-
A car traveling 87.0 km/h is 1500 m behind a truck traveling at 74.0 km/h. How far from its initial position does the car have to travel to catch up to the truck.
-
Calculate the equilibrium constant for the reaction between Ni 2+ (aq) and Cd(s) (at 25 C)
-
Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. a. Pb+ (aq) + Mg(s) b. Br() + 2 CI (aq) c. MnO(s) + 4H+ (aq) + Cu(s) Pb(s) + Mg2+ (aq) 2 Br (aq) + Cl(8) 2+ Mn+ (aq) +...
-
Refresh produces soft drinks and sodas. Production of 103,000 liters was started in February, 88,000 liters were completed. Material costs were $52,020 for the month while conversion costs were...
-
Trico Inc's credit rating is AA. Corresponding credit spread for companies rated AA is 0.78%. If risk-free rate is 1%, what is the cost of debt for Trico Inc? [enter answer in %] Blueberry Inc...
-
A water tank has a maximum capacity of 600 m however internal corrosion is responsible for significant leakage is assess that a constant half cubic meter of water leaks every minute. If a 3 inch...
-
Come home incorporated sold some office equipment for 52000 on december 31 2018 the original cost of the equipment was 80000 with a residual value of 5000 and a useful life of 10 years assume the...
-
Is managerial accounting is more important for small businesses versus large businesses? Why or why not
-
Exactly five years ago, AAA Corporation issued 20-year bonds with a $1,000 face value. These bonds pay $80 in coupon payment every six months. The bonds currently sell for $1,150. Due to additional...
-
Home Improvement Company, a retail home store, has two major divisionsoutdoor and indoor. Here is the data on their income and expenses: Due to the loss, the general manager is considering closing...
-
A test car is driven a fixed distance of n miles along a straight highway. (Here n Z+.) The car travels at one mile per hour for the first mile, two miles per hour for the second mile, four miles...
-
Prove that the equation when applied to a transmission grating, is independent of the refractive index. a(sin 0)m sin ;) ^ [10.61]
-
A grating has a total width of 10.0 cm and contains 600 lines/mm. What is its resolving power in the second-order spectrum? At a mean wavelength of 540 nm, what wavelength difference can it resolve?
-
A high-resolution grating 260 mm wide, with 300 lines per millimeter, at about 75 in autocollimation has a resolving power of just about 10 6 for = 500 nm. Find its free spectral range. How do these...
-
An analyst gathers the following information regarding Finesse Capital. Current price = $54 Current dividend = $1.80 Short-term supernormal growth rate = 12% Long-term sustainable growth rate = 3%...
-
BS Ltd provides consultancy services to small and medium sized businesses. Three types of consultants are employed offering administrative, data processing and marketing advice respectively. The...
-
Create a simple calculator program that can perform addition, subtraction, multiplication, and division operations based on user input

Study smarter with the SolutionInn App


