Calculate the standard enthalpy of reaction for reducing the different forms of iron oxide to iron metal
Question:
Calculate the standard enthalpy of reaction for reducing the different forms of iron oxide to iron metal and CO2 from the reaction of the oxide with CO. Identify which reaction is the most exothermic per mole of iron and explain why.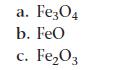
Transcribed Image Text:
a. Fe3O4 b. FeO c. Fe₂O3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
b 110 kJmol a 136 kJmol c 248 kJ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpy of atomization of an element is the energy required to convert one mole of an element in its most stable form at 25C to one mole of monatomic gas. Given that the standard...
-
Calculate the standard enthalpy change, Ho, for the following reaction at 25oC. Fe2O3(s) + 2Al(s) 2Fe(s) + Al2O3(s) What is the enthalpy change per mole of iron?
-
Calculate the standard enthalpy change, Ho, for the following reaction at 25oC. 3CaO(s) + 2Al(s) 3Ca(s) +Al2O3(s) What is the enthalpy change per mole of calcium?
-
In Exercises find the second derivative of the function. f(x) = x + 3x-3
-
At the end of its first year of operations, a company calculated its ending merchandise inventory according to three different accounting methods, as follows: FIFO, $95,000; average-cost, $90,000;...
-
Educational Toys, Inc. (ETI) has highly seasonal sales and financing requirements. The companys balance sheet on December 31, 2005 (now) is as follows: ETI has made the following projections of its...
-
Tyler Company reported the following costs on its financial statements (in thousands): REQUIRED: Using the reserve disclosure for Tyler Company in problem 13 and the data presented in this problem,...
-
Paige Corporation makes a mechanical stuffed alligator that sings the Martian national anthem. The following information is available for Paige Corporations anticipated annual volume of 500,000...
-
Ports should also be able to adapt to global and regional political or social changes. Historically, it is apparent that unexpected sociopolitical developments have demanded ports to change their...
-
Balance the equation for the production of acetylene (C 2 H 2 ) from the reaction of calcium carbide with water. If the acetylene is burned to form water and carbon dioxide, how many kilojoules of...
-
Use the data in Appendix II, Table B to calculate H for the formation of a 1 M solution of H 2 SO 4 from SO 3 (g). B. Standard Thermodynamic Quantities for Selected Substances at 25 C Substance AH...
-
Provide a critical argument for and against the view that Multi National corporations (MNCs) promote convergence of employment practices across Europe. You should draw upon PERMUTTER (1969)...
-
Perform a SWOT analysis of an existing international firm relating to a new host country market. Explain how the firm could capitalize on its strengths and opportunities and overcome its weaknesses...
-
1. What is the study of government, business, and society all about? 2. Why is it important for students to study thus topic? 3. What is meant by the business environment. Discuss. 4. What type of...
-
Q1- what is navigational aid and the three types of navigation 1- non-directional radio beacon (NDB) 2- very high frequency omnidirectional range radio beacon (VOR) 3- instrument landing system...
-
Case Synopsis: Chabot Wallpaper Chabot is a privately owned company which designs, markets, and manufactures wallpaper. The company's products are shipped all over the country under the Chabot brand....
-
ABC Company intends to modernize its factory in the coming year, incurring an immediate renovation expenditure of $50,000. They anticipate receiving $30,000 in payments over the following two years....
-
Is it possible that two different companies both of which have the same amount of assets and generated the exact same amount of revenues and are audited by the same accounting firm could have...
-
Define deferred revenue. Why is it a liability?
-
A positively charged particle moves as shown in Figure P20.40. Which statement best describes the direction of B(vector)? (a) It is into the plane of the figure. (b) It is out of the plane of the...
-
Figure P20.41 shows three identical particles, each with a positive charge and all moving at the same speed. In which case is B(vector) largest? Smallest? Assume B(vector) is perpendicular to the...
-
A charged particle moves as shown in Figure P20.42. Is the particle positively charged or negatively charged? Figure P20.42
-
Attend a school board meeting for a local school district.If you are unable to attend in person, you may watch a live stream or an officialrecorded video of a recent school boardmeeting. In a...
-
1- WHat do you think of this? Amazing presentation.The Burberry noose incident, as well as other similar incidents in the fashion industry, highlight the importance of ethical considerations and...
-
the importance of precedence what legal authority is, and which authority is the most important how to conduct legal research and learn how to use the 4 most effective legal research tools found in...

Study smarter with the SolutionInn App


