Consider the given acid ionization constants. Identify the strongest conjugate base. Acid HNO(aq) HCHO(aq) HCIO(aq) HCN(aq) a)
Question:
Consider the given acid ionization constants. Identify the strongest conjugate base.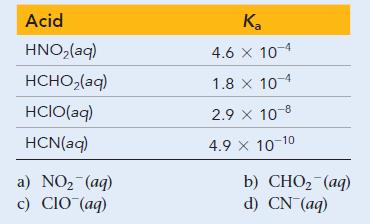
Transcribed Image Text:
Acid HNO₂(aq) HCHO₂(aq) HCIO(aq) HCN(aq) a) NO₂ (aq) c) CIO (aq) Ka 4.6 x 10-4 1.8 x 10-4 2.9 X 10 8 4.9 × 10-10 b) CHO, (aq) d) CN (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
d...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose a simple random sample of size n=64 is obtained from a population that is skewed right with = 72 and = 16. (a) Describe the sampling distribution of x. (b) What is P (X>75.5)? (c) What is P...
-
Which of the following would not be acceptable for performing an insulation resistance test? a)digital multifunctional meter b)digital multimeter c)analogue insultaion resistance tester d)digital...
-
If 100 mol methanol and 100 mol acetic acid are fed to a batch reactor and allowed to come to equilibrium. How many moles of each component will be present in the product stream?
-
Given a circularly linked list L containing an even number of nodes, describe how to split L into two circularly linked lists of half the size.
-
A manufacturing plant has the theoretical capability to produce 54,000 printers per quarter but currently produces 20,250 units. The conversion cost per quarter is $2,430,000. There are 13,500...
-
The lead screw (also called a power screw or a jack screw) is used to convert the rotation of a motor shaft into a translational motion of the mass m (see Figure). For one revolution of the screw,...
-
The Phoenix Corporation reported a net loss but a positive cash flow from operations. Further, despite the net loss, the company continued to pay its regular dividend. Discuss why it is possible for...
-
Better Experts Ltd. (BEL) specializes in taking underperforming companies to a higher level of performance. BELs capital structure at December 31, 2011, included 10,000 shares of $2.25 preferred...
-
8- In this step, do below configuration on Pfsense: Go to firewall/rules/WAN and define a new rule to pass all the ICMP traffic from WAN side to LAN side
-
What is a carboxylic acid? Give an example.
-
Consider the three generic weak acids HA, HB, and HC. The images shown here represent the ionization of each acid at room temperature. Which acid has the largest Ka? (a) HA (b) HB (c) HC
-
Solve the right triangles with the given parts or state that there is not enough information to solve. Round off results according to Table 4.1. Refer to Fig. 4.37. B = 32.1, c = 238 Data from Table...
-
Determine the cost of the project and the revenues from the project for the period management wishes to analyze. For example, a firm wants to start a new widget plant. The cost of the plant is...
-
Texas Chemicals is a major producer of oil-based fertilisers in the US.The company's stock is currently selling for $80 per share and there are 10 million shares outstanding. The company also has...
-
Peace Corporation just paid a dividend of $1.05 a share (Do = $1.05). The dividend is expected to grow by 6% annually for the foreseeable future. What is the expected value of the dividend 1 year...
-
The errors of the forecasts have been calculated using 4 methods, which are shown below: Method Average Error MAD A)3-month moving average 0.7 7.40 B) Moving average 3-month weighted -0.3 8.40...
-
2. Suppose your company provides a $50,000 three-year loan to the partner at a variable rate. The initial variable is 12%. The initial funds come from two sources: own savings $25,000 (assuming zero...
-
Heres a plot of the Studentized residuals from the regression model of Exercise 18 plotted against ArterialMPH. The plot is colored according to City Size (Small, Medium, Large, and Very Large), and...
-
What is a lobbyist in US? How did this term emerge?
-
A farsighted person can see very distant mountains with relaxed eyes while wearing +3.0D contact lenses. Prescribe spectacle lenses that will serve just as well when worn 17 mm in front of the...
-
A person who is farsighted has her near point at 100 cm and her far point is where it should normally be. Determine the prescription for a contact lens that will fix the problem. Locate her new far...
-
A 6 D myope has a far point 16.67 cm from the eye. Prescribe a spectacle lens to be worn 12 mm from the eye that will correct his vision.
-
4. Congratulations! You completed your accounting education and have now been hired by a CPA firm as a new staff auditor. The Senior Partner has assigned you to an audit of The Neighborhood Shoppe, a...
-
A distributor use a Periodic Review Policy and places order every 3 weeks for Galaxy NoteVI, the Lead time is 2 weeks. The Average weekly demand is 44.58; standard deviation of weekly demand is...
-
US GAAP provides the "rulebook" to help ensure all for-profit and not-for-profit entities present their _____ accurately. Select an answer: financial performance results taxes governmental records...

Study smarter with the SolutionInn App


