Consider the reaction: Calculate G rxn for the reaction at 25 C under each of the following
Question:
Consider the reaction:
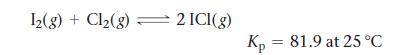 Calculate ΔGrxn for the reaction at 25 °C under each of the following conditions:
Calculate ΔGrxn for the reaction at 25 °C under each of the following conditions:
a. Standard conditions
b. At equilibrium
c. PICl = 2.55 atm; PI2 = 0.325 atm; PCl2 = 0.221 atm
Transcribed Image Text:
12(g) + Cl₂(g) = 2 ICI(g) Kp = 81.9 at 25°C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The standard Gibbs free energy change AG ...View the full answer

Answered By

John Kago
Am a processional practicing accountant with 5 years experience in practice, I also happens to have hands on experience in economic analysis and statistical research for 3 years. am well conversant with Accounting packages, sage, pastel, quick books, hansa world, etc, I have real work experience with Strata, and SPSS
4.70+
31+ Reviews
77+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the reaction: Calculate G rxn for the reaction at 25 C under each of the following conditions: a. Standard conditions b. At equilibrium c. P CH3OH = 1.0 atm; P CO = P H2 = 0.010 atm CO(g) +...
-
Ray Holt Corporation has retained you as a consultant on accounting policies and procedures. During 2019, the company engaged in a number of treasury stock transactions, having foreseen an...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
What problems may be encountered in making a comparative study of remuneration reports?
-
What is the difference between an activity flexible budget and a functional-based (traditional) flexible budget?
-
1. Ignore the problem. Jacobss contributions to new product development are too valuable to risk losing him, and the problems over the past 10 years have always worked themselves out anyway. No sense...
-
If your instructor assigns a marketing plan for your class, we hope you will be excitedfor two reasons. First, you will get insights into trying to actually do marketing that often go beyond what you...
-
Presented below are data on three promissory notes. Determine the missingamounts. Date of Note Total Maturity Annual Interest Rate Principal Terms 60 days 30 days 6 months Date Interest (a) April 1...
-
The relational model representation of an EERD relationship with a cardinality of (M,M) must be represented with a relationship table. Explain why this is true.
-
Explain the difference between macrostates (external arrangements of particles) and microstates (internal arrangements of particles).
-
Estimate the value of the equilibrium constant at 525 K for each reaction in Problem 73. Problem 73 Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction....
-
Mack Company is a European subsidiary of Bear Down Corporation, a U.S. company. Mack had the following balance sheets at December 31, 20X2, and December 31, 20X1. Also provided above are the U.S....
-
Projects often have a strategic and innovative focus resulting in using current technology. Why?
-
Delve into the significance of scheduling within the realm of operations management.
-
Create a copy of the June Totals worksheet and name it June 1st Totals. Return to the June Totals worksheet. When printed you would like the titles to be preserved. Set the titles in row 5 and...
-
Assume that Edison Group has invited you as an external consultant to analyse its portfolio of companies. You decide to use the BCG matrix as a tool for the analysis. In which category would you...
-
With respect to the following costs, first identify if the expenditure is a business or investment expenditure. Then, discuss whether each is an expense or capital expenditure. Taxpayer owns and...
-
What professional certifications exist for the audit professional and IT auditor?
-
DC has unused FTC carryover from 2017 in the separate category for GC income as the result of income generated by a foreign branch. The income was foreign source general category income. In 2018 the...
-
Name a type of compressor often used for pneumatic fluid power systems.
-
The intake duct to a fan consists of intake louvers, 5.8 m of square duct (800 800 mm), a sudden contraction to a 400-mm-diameter round duct, and 9.25 m of the round duct. Estimate the pressure at...
-
The electric field of an electromagnetic plane wave is given in SI units by (a) What is the wave?s angular frequency? (b) Write an expression for vector k. (c) What is the value of k? (d) Determine...
-
1. You are the strategy and expansion manager for Econ Electric Scooter. You are evaluating whether to develop a new electric scooter line Bangladesh or to export (scooters produced at headquarters...
-
The market price of a bushel of wheat prior to the price floor was $800. The minimum legal price is now $1,000. In the next growing season, farmers are able to sell 1 billion bushels but end up with...
-
Hi, what would be the account name for this? Provided gardening services to a new client Tony Fletcher and invoiced him $2,100. This invoice is due to be paid by the 25 th May 2023 and the reference...

Study smarter with the SolutionInn App


