Consider this three-step mechanism for a reaction: a. What is the overall reaction? b. Identify the intermediates
Question:
Consider this three-step mechanism for a reaction: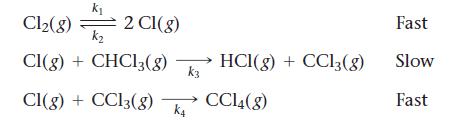
a. What is the overall reaction?
b. Identify the intermediates in the mechanism.
c. What is the predicted rate law?
Transcribed Image Text:
k₁ Cl₂(8) k₂ Cl(g) + CHCl3(8) HC1(g) + CC13 (8) Cl(g) + CC13(g) CC14(8) 2 Cl(g) k3 K4 Fast Slow Fast
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Clg CHCl3...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCl3) and chlorine: Step 1: Step 2: Step 3: (a) What is the overall reaction? (b) What are the intermediates in...
-
A proposed mechanism for a reaction is C4H9Br C4H9+ + Br2 Slow C4H9+ + H2O C4H9OH2+ Fast C4H9OH2+ + H2O C4H9OH + H3O+ Fast Write the rate law expected for this mechanism. What is the overall...
-
In a hydrocarbon solution, the gold compound (CH3)3AuPH3 decomposes into ethane (C2H6) and a different gold compound, (CH3)AuPH3. The following mechanism has been proposed for the decomposition of...
-
Redo Exercise 29 for the situation in which Ms. Jones withdrew $1000 at the end of the seventh year instead of depositing it. Data in Exercise 29 Ms. Jones deposited $100 at the end of each month for...
-
What is cost measurement? Cost accumulation? What is the difference between the two?
-
An object of mass m1 = 9.00 kg is in equilibrium while connected to a light spring of constant k = 100 N/m that is fastened to a wall as shown in Figure P15.52a. A second object, m2 = 7.00 kg, is...
-
A fixed mass of gas emits \(250 \mathrm{~J}\) of heat energy and contracts at a constant pressure of \(1 \times 10^{5} \mathrm{~Pa}\) from \(2.5 \times 10^{-3} \mathrm{~m}^{3}\) to \(1.0 \times...
-
Antibiotic-resistance genes, as well as other virulence factor genes, are easily passed between bacterial cells through horizontal gene transfer. a. Conduct additional research and summarize the...
-
Sombrero Corporation, a U.S. corporation, operates through a branch in Espania. Management projects that the company's pretax income in the next taxable year will be $117,600: $89,600 from U.S....
-
Consider this two-step mechanism for a reaction: a. What is the overall reaction? b. Identify the intermediates in the mechanism. c. What is the predicted rate law? NO(g) + Cl(8) NO(g) + Cl(g) K k...
-
Consider this overall reaction, which is experimentally observed to be second order in X and first order in Y: a. Does the reaction occur in a single step in which X and Y collide? b. Is this...
-
Reduce the equations to slope-intercept form and find the slope and the y-intercept. Sketch each line. 3x 2y 1 = 0
-
Mental health and substance abuse Statement of the Policy Issue A brief statement describing the issue Background related to the issue An explanation of the problem and its significance. Include...
-
There is no spoilage, the beginning WIP Inventory is 10,750 units, and the ending WIP Inventory is 6,400 units for the second department in a two-department process. What is the number of units...
-
During 2022, Cullumber Company sold a building with a book value of $152,000 for proceeds of $182,000. The company also sold long-term investments for proceeds of $39,000. The company purchased land...
-
Gabbe Industries is a division of a major corporation. Last year the division had total sales of $24,049,700, net operating income of $4,641,592, and average operating assets of $8,293,000. The...
-
Morganton Company makes one product, and has provided the following information to help prepare the master budget for its first four months of operations: The budgeted selling price per unit is $60....
-
You have been engaged to audit the financial statements of Quinn Corporation for the year ended December 31, 2010.During the year Quinn obtained a long-term loan from a local bank. The finance terms...
-
2. Assume a person bends forward to lift a load "with his back" as shown in Figure P12.42a. The person's spine piv- ots mainly at the fifth lumbar vertebra, with the principal supporting force...
-
A person tries to cool a kitchen by switching on an electric fan and closing the kitchen door and windows. What will happen?
-
Why are both a hot and a cold reservoir needed for a heat engine to operate?
-
A lead bullet at 100C strikes a steel plate and melts. What was its minimum speed? The specific heat of lead is 0.13 kJ/kg C.
-
Beckman Engineering and Associates (BEA) is considering a change in its capital structure. BEA currently has $20 million in debt carrying a rate of 6%, and its stock price is $40 per share with 2...
-
Assume the market capitalization of risky asset 2 is $1 billion. What is the market capitalization of risky asset 1?
-
Dye Trucking raised $220 million in new debt and used this to buy back stock. After the recap, Dye's stock price is $5.50. If Dye had 50 million shares of stock before the recap, how many shares does...

Study smarter with the SolutionInn App


