Consider this overall reaction, which is experimentally observed to be second order in X and first order
Question:
Consider this overall reaction, which is experimentally observed to be second order in X and first order in Y:![]()
a. Does the reaction occur in a single step in which X and Y collide?
b. Is this two-step mechanism valid?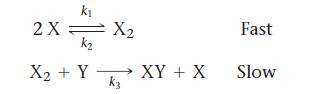
Transcribed Image Text:
X + Y→→→ XY
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a No the reaction does not occur in a single step in ...View the full answer

Answered By

Wahome Michael
I am a CPA finalist and a graduate in Bachelor of commerce. I am a full time writer with 4 years experience in academic writing (essays, Thesis, dissertation and research). I am also a full time writer which assures you of my quality, deep knowledge of your task requirement and timeliness. Assign me your task and you shall have the best.
Thanks in advance
4.90+
63+ Reviews
132+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider this overall reaction, which is experimentally observed to be second order in AB and zero order in C: Is the following mechanism valid for this reaction? AB + C A + BC
-
Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in the reactant, and k1 is known to be 0.250 Lmol-1s-1 at 258C. In a particular experiment...
-
A reaction was found to be second order in carbon monoxide concentration. The rate of the reaction _____ if the [CO] is doubled, with everything else kept the same. Please explain. A) remains...
-
Bob Bristol just called to congratulate you on your excellent work on the various assignments at CII.He now wants you to do some capacity analysis for Meghan Willoughby, the Chief Purser. Meghan is...
-
Young, Coopers, and Touche (YCT) is a tax services firm. The firm is located in San Diego and employs 10 professionals and eight staff. The firm does tax work for small businesses and well-to-do...
-
Timo Companys standard materials cost per unit of output is $10 (2 pounds $5). During July, the company purchases and uses 3,200 pounds of materials costing $16,160 in making 1,500 units of finished...
-
Identify at least three grounds a party may have for objecting to interrogatories.
-
On December 31, 2018, Mainland Corporation issues 6%, 10-year convertible bonds payable with a face value of $4,000,000. The semiannual interest dates are June 30 and December 31. The market interest...
-
Why and how to identify the most valuable customers and maximize their customer lifetime value (CLV)?
-
Consider this three-step mechanism for a reaction: a. What is the overall reaction? b. Identify the intermediates in the mechanism. c. What is the predicted rate law? k Cl(8) k Cl(g) + CHCl3(8)...
-
From a process, 100 kg of steam is available at 2 MPa and 800C. a. Determine the maximum amount of shaft work that can be obtained from this steam in a non-flow process if the ambient conditions are...
-
For this question, use the spreadsheet "multinational employment.xls." (a) Recall that Slaughter made a point that over the course of his data, "for every one job that U.S. multinationals created...
-
A craft coffee shop located in the lobby of a downtown skyscraper sells a cup of coffee for $ 6 . 0 0 . Employees who work in a building three blocks away have an easy access to a food truck that...
-
Generate a half page- one page report for each topic about Dutch Bros Page 1: Brand awareness, brand association, and brand personality Page 2: Competition Analysis Page 3: Current Marketing...
-
An arc-welding operation on nickel performs a groove weld, whose cross-sectional area = 29 mm 2 . Travel velocity = 3.9 mm/s. Heat transfer factor = 0.83 and melting factor = 0.64. Determine the rate...
-
In project management, organisational structure plays a vital role in determining how projects are planned, executed, and controlled. Unlike traditional hierarchical structures, the matrix structure...
-
Relate the Gaps model to some of the examples from the session 1 discussion. Which gaps (or lack thereof) contributed to either good or bad service experiences? Which gaps are the most or least...
-
Suppose Treasury bills (face value of $10,000) with maturities of 30 days, 90 days, and 180 days sell at the respective annualized discounts of 4.25%, 4.35%, and 4.92%. What are the respective money...
-
Suppose that you could invest in the following projects but have only $30,000 to invest. How would you make your decision and which projects would you invest in? Project Cost $ 8,000 11,000 9,000...
-
Many power stations get rid of their waste heat by using it to boil water and allowing the resulting steam to escape into the atmosphere via a cooling tower. How much water would a power station need...
-
How much heat is given off when 1 kg of steam at 100C condenses and cools to water at 20C?
-
If you wish to speed up the rate at which potatoes are cooking in a pan of boiling water, would it be better to turn up the gas flame or use a pressure cooker?
-
Your attorney, Barry Smart, comes to you looking a bit exasperated and confirms with you that client, Larry Last, is coming in tomorrow with his witnesses to sign his Last Will and Testament. You...
-
The trial balances for Wallace Corporation and Au Inc. at December 31, Year 4, just before the transaction described below, were as follows: Required: What are the balances for the land, other...
-
Proponents of this view argue that an unequal distribution of rewards, such as higher incomes or profits, can provide individuals with incentives to work harder, invest, and take risks. The prospect...

Study smarter with the SolutionInn App


