Ethene (C 2 H 4 ) can be halogenated by this reaction: where X 2 can be
Question:
Ethene (C2H4) can be halogenated by this reaction:![]()
where X2 can be Cl2 (green), Br2 (brown), or I2 (purple). Examine the three figures representing equilibrium concentrations in this reaction at the same temperature for the three different halogens. Rank the equilibrium constants for the three reactions from largest to smallest.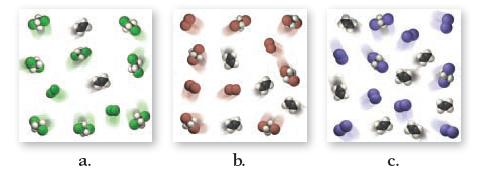
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





