Find E cell for an electrochemical cell based on the following reaction with [MnO 4 ] =
Question:
Find Ecell for an electrochemical cell based on the following reaction with [MnO4¯] = 2.0 M, [H+] = 1.0 M, and [Ag+] = 0.010 M. E°cell for the reaction is +0.88 V.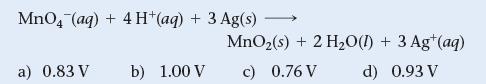
Transcribed Image Text:
MnO4 (aq) + 4H+ (aq) + 3 Ag(s) a) 0.83 V b) 1.00 V - MnO₂ (s) + 2 H₂O(1) + 3 Ag+ (aq) c) 0.76 V d) 0.93 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
b...View the full answer

Answered By

Vincent Omondi
I am an extremely self-motivated person who firmly believes in his abilities. With high sensitivity to task and operating parameters, deadlines and keen on instructions, I deliver the best quality work for my clients. I handle tasks ranging from assignments to projects.
4.90+
109+ Reviews
314+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Given the circuit in Fig. 8.95, find i(t) and v(t) for t > 0. 1 H 2 0 6 V
-
Determine the cell potential for an electrochemical cell based on the following two half-reactions: Oxidation: Cu(s)- >>> Cu+ (aq, 0.010 M) + 2 e Reduction: MnO4 (aq, 2.0 M) + 4 H(aq, 1.0 M) + 3 e...
-
Consider an electrochemical cell based on the half-reactions Ni 2+ (aq) + 2 e Ni(s) and Cd 2+ (aq) +2 e Cd(s). (a) Diagram the cell, and label each of the components (including the anode,...
-
Daniel and Karen Chapman have three children, aged 2, 8 and 11 at the end of the year. The 8 year old is blind and therefore qualifies for the disability tax credit. The other two are in good mental...
-
Indicate whether each of the following is a characteristic of job order costing or of process costing: 1. Several Work in Process Inventory accounts are used, one for each department or work cell in...
-
Roberto Martinez was the sole force behind In Over Our Heads, Inc., a corporation designed to run a year-round community swimming pool. The enterprise was incorporated in the correct manner in...
-
The enzyme lipase catalyzes the hydrolysis of esters of fatty acids. The hydrolysis of p-nitrophenyloctanoate was followed by measuring the appearance of p-nitrophenol in the reaction mixture: The...
-
Presented below is an amortization schedule related to Spangler Company??s 5-year, $100,000 bond with a 7% interest rate and a 5% yield, purchased on December 31, 2010, for $108,660. The following...
-
Who is Tesla's auditor? Did Tesla's receive a "clean" (unmodified) audit opinion? How many critical audit matters were discussed in Tesla's audit report?
-
Gold can be plated out of a solution containing Au 3+ according to the half-reaction: What mass of gold (in grams) is plated by a 25-minute flow of 5.5 A current? 3+ Au+ (aq) + 3 e Au(s)
-
Which statement is true for both electrolytic and voltaic cells? (a) The cell spontaneously produces a positive voltage. (b) Electrons flow from the anode to the cathode. (c) Oxidation occurs at the...
-
Darlington Foods is an integrated wholesaler and retailer of high-quality food products. It provides gourmet foods to supermarket chains and specialty stores in the United States and Europe under the...
-
Edward started a business as a primary producer in Year 1. Trading results until the current year are as follows: Year ended 30 June Trading profit $ Year 1 Profit 8,000 Year 2 Profit 6,000 Year 3...
-
John purchased 300 shares of Big Trouble common stock at $26.75 a share in July of 2015. In June of 2016 John buys 3 October 35 puts at 3. If the market of Big Trouble is trading at $33.16 at...
-
The cash flows associated with an investment project are an immediate cost of $1000 and benefits of $1800 in one year, $1400 in two years, and $1400 in three years. The cost of capital (WACC) is 10%....
-
The following information pertains to Alpha Projects for the quarter ended 31 December: Actual Budgeted November December October R R R Invoices (20% for cash and 80% on credit) 360 000 380 000 400...
-
Brandy Corporation, a calendar-year, accrual-basis taxpayer, signed a contract on December 27, 20X1, for the installation of a new central air conditioning unit. Because of various shipping delays,...
-
Describe some of the ways the Internet has or has not impacted the way you, (1) Make travel plans, (2) Read news, (3) Decide which movie to see next.
-
A researcher reports a significant two-way between-subjects ANOVA, F(3, 40) = 2.96. State the decision to retain or reject the null hypothesis for this test.
-
Prove that the area under the convolution of the functions (x) and h(x) equals the product of the areas under each of those functions.
-
Examine the three graphs in Fig. P. 11.20 and explain whats being illustrated. Discuss how the shape of g(X) arises. Why is g(X) symmetrical about X = 0? Whats the significance of the width of g(x)?...
-
Prove analytically that the convolution of any function (x) with a delta function, (x), generates the original function (X).
-
4. Alice was having a conversation with her friend Trina, who had a discovery to share: Pick any two integers. Look at the sum of their squares, the difference of their squares, and twice the product...
-
Suppose this is a stock-for-stock deal. Instead of purchasing Target's stock with cash, Walmart offers to give every Target shareholder share of Walmart for each share of Target (and Walmart is...
-
Two hundred items were purchased at a list price of $120 each less trade discounts of 25/15. A markup of 60% based on sales was used to determine the initial selling price. a) Determine the initial...

Study smarter with the SolutionInn App


