For each of the reactions, calculate the mass (in grams) of the product that forms when 3.67
Question:
For each of the reactions, calculate the mass (in grams) of the product that forms when 3.67 g of the underlined reactant completely reacts. Assume that there is more than enough of the other reactant.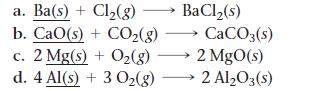
Transcribed Image Text:
a. Ba(s) + Cl₂(g) b. CaO(s) + COz(g) CaCO3(s) c. 2 Mg(s) + O₂(g) →→→ 2 MgO(s) d. 4 Al(s) + 3 O₂(g) 2 Al₂O3(s) BaCl₂(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 556 g Ba...View the full answer

Answered By

SILPA MARY THOMAS
I have done my graduation and post-graduation in Chemistry. I have one year research experience and 4+ years teaching experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the reactions, calculate the mass (in grams) of the product that forms when 15.39 g of the underlined reactant completely reacts. Assume that there is more than enough of the other...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Hernandez Company began 2010 with a $120,000 balance in retained earnings. During the year, the following events occurred: 1. The company earned net income of $80,000. 2. A material error in net...
-
Romo, Inc., has current assets of $1,850, has current assets of $1,850, net fixed assets of $8,600, current liabilities of $1,600, and long-term debt of $6,100. What is the value of the shareholders...
-
In the first stage of a paper drying process, a cylinder of diameter 0.15 m is covered by moisture-soaked paper. The temperature of the paper is maintained at 70C by imbedded electrical heaters. Dry...
-
A construction contract has the following language: It is the responsibility of the contractor to inspect and become familiar with the Project and to acquaint itself thoroughly with all conditions...
-
Singleton Supplies Corporation (SSC) manufactures medical products for hospitals, clinics, and nursing homes. SSC may introduce a new type of X-ray scanner designed to identify certain types of...
-
(d) What is the value of "j" after the execution of the partial code in Figure 4.2? Explain your answer. int j = 10; for (int i = 0; 0; i
-
Assume that you are the management accountant at Zelda Limited, a manufacturer of cables for the telecommunication industry. During the coffee break, you are having a conversation with Sandy, another...
-
Find the limiting reactant for each initial amount of reactants. a. 2 mol Na, 2 mol Br 2 b. 1.8 mol Na, 1.4 mol Br 2 c. 2.5 mol Na, 1 mol Br 2 d. 12.6 mol Na, 6.9 mol Br 2 2 Na(s) + Br(g) Br(g) 2...
-
Sulfuric acid dissolves aluminum metal according to the reaction: Suppose you want to dissolve an aluminum block with a mass of 15.2 g. What minimum mass of H 2 SO 4 (in g) do you need? What mass of...
-
When the lunar excursion module (LEM) was set adrift after returning two of the Apollo astronauts to the command module, which was orbiting the moon at an altitude of 87 mi, its speed was reduced to...
-
Do judges ever confer with law enforcement to assist in determining an appropriate sentence? Please explain
-
do victims get a say in whether a plea bargain should be offered in a particular case? Please explain
-
Briefly explain the Mapp v Ohio 1961 court case and how this relates to the violation of the fourth amendment and how this is of importance to our culture now?
-
Do lenient sentences ultimately harm society? Please explain
-
Create a class named Yourlastname_Yourfirstname_hw4. (10 pts) 1. Add a method named problem1 that keeps asking the user for words and joins them together. Each word should start with an uppercase...
-
The cash account for Leisure Systems at February 29, 2012, indicated a balance of $4,720. The bank statement indicated a balance of $18,650 on February 29, 2012. Comparing the bank statement and the...
-
Give an example of transitory income. What effect does this income have on the marginal propensity to consume?
-
When 2-ethyl-5-chlorotoluene was treated with sodium hydroxide at high temperature, followed by treatment with H 3 O + , three constitutional isomers with molecular formula C 9 H 12 O were obtained....
-
You wish to design an effusion source for Br atoms from Br 2 (g). If the source is to operate at a total pressure of 7.5 Torr, what temperature is required to produce a degree of dissociation of...
-
Calculate G for the isothermal expansion of 2.25 mol of an ideal gas at 325 K from an initial pressure of 12.0 bar to a final pressure of 2.5 bar.
-
A beam of light travels from a vacuum into water at an angle of 45. The light has a frequency of 6.00 x 1014 Hz and travels at a speed of 2.26 x 108 m/s in water. The speed of light in a vacuum is...
-
31 g (20%) A three-mirror ring cavity has mirrors with R = R = 0.8 and R = 0.9 which are separated at 12 = 0.5 m and 123 = 3 = 0.3 m. A glass rod that has a length of 1 = 0.2 m and a refractive index...
-
Car Crash Investigation Background information: A collision occurred involving two vehicles on Route 28 N. The speed limit in this zone is 45 mph. A 2011 Honda Odyssey minivan was stopped at the...

Study smarter with the SolutionInn App


