One way to evaluate fuels with respect to global warming is to determine how much heat they
Question:
One way to evaluate fuels with respect to global warming is to determine how much heat they release during combustion relative to how much CO2 they produce. The greater the heat relative to the amount of CO2, the better the fuel. Use the combustion reactions of carbon, natural gas, and octane, in combination with the enthalpy of combustion for each reaction (all given earlier), to calculate the heat (in kJ) released by each fuel per 1.00 kg of CO2 produced.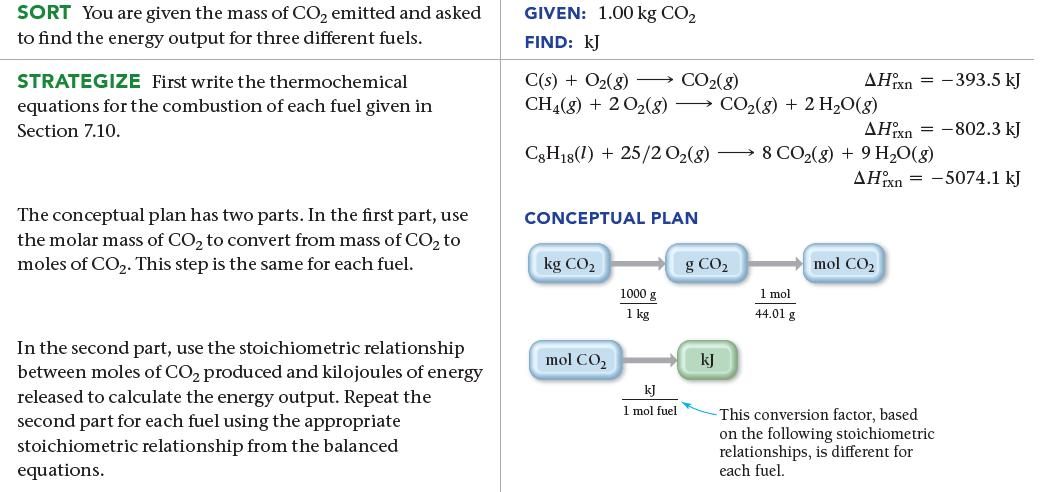

Transcribed Image Text:
SORT You are given the mass of CO₂ emitted and asked to find the energy output for three different fuels. STRATEGIZE First write the thermochemical equations for the combustion of each fuel given in Section 7.10. The conceptual plan has two parts. In the first part, use the molar mass of CO₂ to convert from mass of CO₂ to moles of CO₂. This step is the same for each fuel. In the second part, use the stoichiometric relationship between moles of CO₂ produced and kilojoules of energy released to calculate the energy output. Repeat the second part for each fuel using the appropriate stoichiometric relationship from the balanced equations. GIVEN: 1.00 kg CO₂ FIND: KJ C(s) + O₂(g) → CO₂(g) CH4(g) + 2O2(8) CO₂(g) + 2 H₂O(g) C8H18(1) + 25/2 02(8) → 8 CO₂(g) + 9 H₂O(g) CONCEPTUAL PLAN kg CO₂ mol CO₂ 1000 g 1 kg kJ 1 mol fuel g CO₂ kJ AHixn = 1 mol 44.01 g AHixn = -802.3 kJ -393.5 kJ AHixn=-5074.1 kJ mol CO₂ This conversion factor, based on the following stoichiometric relationships, is different for each fuel.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
100 kg CO X 1000 g 1 kg X For C 2272 mol C...View the full answer

Answered By

Lamya S
Highly creative, resourceful and dedicated High School Teacher with a good fluency in English (IELTS- 7.5 band scorer) and an excellent record of successful classroom presentations.
I have more than 2 years experience in tutoring students especially by using my note making strategies.
Especially adept at teaching methods of business functions and management through a positive, and flexible teaching style with the willingness to work beyond the call of duty.
Committed to ongoing professional development and spreading the knowledge within myself to the blooming ones to make them fly with a colorful wing of future.
I do always believe that more than being a teacher who teaches students subjects,...i rather want to be a teacher who wants to teach students how to love learning..
Subjects i handle :
Business studies
Management studies
Operations Management
Organisational Behaviour
Change Management
Research Methodology
Strategy Management
Economics
Human Resource Management
Performance Management
Training
International Business
Business Ethics
Business Communication
Things you can expect from me :
- A clear cut answer
- A detailed conceptual way of explanation
- Simplified answer form of complex topics
- Diagrams and examples filled answers
4.90+
46+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Multinational oil company ExxonMobil faced many challenges related to climate change. Climate change is taking place because of the greenhouse effect. When solar radiation passes through the...
-
In solar-heated buildings, energy is often stored as sensible heat in rocks, concrete, or water during the day for use at night. To minimize the storage space, it is desirable to use a material that...
-
Most businesses will incur debt at some point during their existence. Question 26 options: True False
-
Sharpton Fabricators Corporation manufactures a variety of parts for the automotive industry. The company uses a job-order costing system with a plantwide predetermined overhead rate based on direct...
-
The velocity of a particle is given by v = 6t + 3, where t is in seconds and v is in meters per second. (a) Sketch v(t) versus t, and find the area under the curve for the interval t = 0 to t = 5 s....
-
A European recession and the U.S. economy a. In 2014, European Union spending on U.S. goods accounted for \(18 \%\) of U.S. exports (see Table 17-2), and U.S. exports amounted to \(15 \%\) of U.S....
-
The following events were completed by Chan's Imports in September 2012. Sept. 1 Acquired $60,000 cash from the issue of common stock. 1 Purchased $36,000 of merchandise on account with terms 2/10,...
-
Breast cancer patients in a London, England, hospital were being treated for spinal metastases. They were followed over a five-year period, with their ambulatory state being recorded before treatment...
-
What is heat? Explain the difference between heat and temperature.
-
A city of 100,000 people uses approximately 1.0 * 10 11 kJ of energy per day. Suppose all of that energy comes from the combustion of liquid octane (C 8 H 18 ) to form gaseous water and gaseous...
-
According to a news story about the bus system in the Lehigh Valley in Pennsylvania, Ridership fell 14 percent in 2012 after a 33 percent increase in bus fares. Based on this information, is the...
-
Can a delivery system with multiple providers have more than one risk pool? Explain your answer.
-
In a game, a player rolls two dice and counts how many points he gets between them. Write out the sampling distribution.
-
Suppose you draw two cards from a standard 52-card deck with replacement. Write out the sampling distribution for the suit drawn.
-
A cigarette manufacturer came up with a new brand of cigarettes called Long Life. The nicotine content of the cigarettes follows a normal distribution with a mean of 20 and a standard deviation of 5....
-
This case study explores how the reach and influence of social media is changing and reshaping the marketing environment. It introduces the Social Media Marketing Framework (SMMF) as a tool to review...
-
Tomasco, Inc., began operations in January 2006 and had the following reported net income or loss for each of its five years of operations: 2006 .......$ 150,000 loss 2007 ....... 130,000 loss 2008...
-
Tiger, Inc. signed a lease for equipment on July 1, 2007.The lease is for 10 years (the useful life of the asset).The first of 10 equal annual payments of $500,000 was made on July 1, 2007.The...
-
Initially, gaps between the A-36 steel plate and the rigid constraint are as shown. Determine the normal stresses Ï x and Ï y in the plate if the temperature is increased by T = 100°F....
-
The steel shaft has a radius of 15 mm. Determine the torque T in the shaft if the two strain gages, attached to the surface of the shaft, report strains of ε x² = -80(10 -6 ) and...
-
The shaft has a radius of 15 mm and is made of L2 tool steel. Determine the strains in the x² and y² direction if a torque T = 2 kN · m is applied to the shaft. 45 VT
-
discuss the ethical dimensions of organizational culture, including the role of ethical leadership, moral values, and ethical decision-making frameworks in promoting a culture of integrity,...
-
Windsor Company adopted a stock-option plan on November 30, 2024, that provided that 67,900 shares of $5 par value stock be designated as available for the granting of options to officers of the...
-
A credit union's Rate-Climber GIC pays rates of 3%, 3.5%, and 4% compounded semiannually in successive years of a three-year term How much interest will be earned in the second year, if $13,000 is...

Study smarter with the SolutionInn App


