Pick an appropriate solvent from Table 14.3 to dissolve each substance. State the kind of intermolecular forces
Question:
Pick an appropriate solvent from Table 14.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case.
a. Motor oil (nonpolar)
b. Ethanol (polar, contains an OH group)
c. Lard (nonpolar)
d. Potassium chloride (ionic)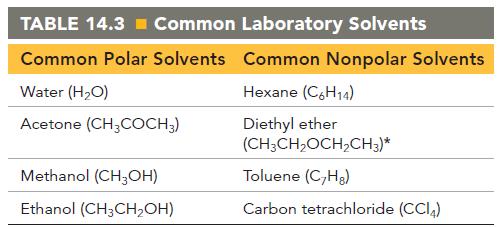
Transcribed Image Text:
TABLE 14.3= Common Laboratory Solvents Common Polar Solvents Common Nonpolar Solvents Hexane (C6H14) Diethyl ether (CH₂CH₂OCH₂CH3)* Toluene (C₂Hg) Carbon tetrachloride (CC14) Water (H₂O) Acetone (CH3COCH3) Methanol (CH3OH) Ethanol (CH3CH₂OH)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a Hexane toluene or CCl 4 dispersion for...View the full answer

Answered By

AJIN kuriakose
I have completed B.Tech in Electrical Engineering & Masters in Power & Control From one of the best universities in India. I got the 99.05 percentile in the Gate Electrical Engineering Exam. I can Help students solving assignments in Electrical subjects like Power Electronics, Control system, Analog, Network Theory & Engineering Mathematics. Clear your fundamentals and develop problem-solving skills and analytical skills to crack the exam.
Get guidance and the opportunity to learn from experienced...
I can provide tuition for Electrical engineering subjects (Power Electronics, Digital electronics, Network Theory, Control System & Engineering Mathematics). The toughest subject of Electrical engineering can be made simple in online classes...
I can also solve it.
1 .I can help you with your assignments or exams or quiz or tutoring.
2. Very strict to the deadlines.
Message me for any help in assignments, live sessions. I am here to help students for all assignments, tests and exams and I will make sure you always get _95% In your subject.
Contact me in solution inn for any help in your semester, projects and for many more things . Also feel free to contact me through solution inn and for any advise related to tutoring and how it works here.thank you.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Pick an appropriate solvent from Table 14.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. a. Isopropyl alcohol...
-
A.) Pick an appropriate solvent to dissolve acetic acid (polar,contains an OH group) . Water (H 2 O) Acetone (CH 3 COCH 3 ) Methanol (CH 3 OH) Ethanol (CH 3 CH 2 OH) Hexane (C 6 H 14 ) Diethyl...
-
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that would occur between the solute and the solvent in which the molecule is...
-
Dwights preferences over beer and whiskey satisfy more is better, but are concave (thus violating the usual assumption of convexity). a. On a diagram, sketch what such indifference curves would look...
-
Hemple produces a variety of pocket PCs. Due to competitive pressures, the company is implementing an activity-based management (ABM) system with the objective of reducing costs. ABM focuses...
-
The following circuit operates if and only if there is a path of functional devices from left to right. The probability each device functions is as shown. Assume that the probability that a device...
-
What is TME? Give examples.
-
Kerwick Company had accounts receivable of $100,000 on January 1, 2014. The only transactions that affected accounts receivable during 2014 were net credit sales of $1,000,000, cash collections of...
-
You are a data analyst working for a nonprofit organization that reviews business. You have been tasked to provide a briefing on consumer complaints. Using this website...
-
What keeps the particles in a colloidal dispersion from coalescing?
-
What is the Tyndall effect, and how can it be used to help identify colloidal dispersions?
-
Define reliability. Explain its role in quality control and improvement.
-
What is a personality trait you have in common with your parents? Perhaps it is a reaction you have in certain situations or something you find yourself saying. Is it a good or bad thing you picked...
-
Discuss how men and women differ in their efforts to end their lives. How do marriage, age, and ethnicity influence these differences? 3. Discuss suicide prevention strategies.
-
Reference the article on Workplace Wellness Programs Workplace Wellness Programs - Alternative Formats , which provides an example of a complete policy. Compile a concise model policyon opioid...
-
Newton's Universal Law of Gravity (NULG) A) Discuss Newton's Universal Law of Gravity (NULG). a. What is required for there to be gravity? b. On what does gravity depend? c. What is the difference,...
-
How you and management at your organization could reduce the potential impact of a strike? Explain in details.
-
Stead Corporation is formed in Year 4 to take over the operations of a small business. This business proved very stable for Stead, as is evidenced here ($ in thousands): Stead also expends $1,400,000...
-
If the cylinder described in Problem 21.3 were initially heated to 500F, how long would it take for the center of the cylinder to cool to 240F if it were constructed of a. Copper? b. Brass? c. Nickel?
-
A car is moving with a velocity of 20 m/s when the brakes are applied and the wheels lock (stop spinning). The car then slides to a stop in 40 m. Find the coefficient of kinetic friction between the...
-
You are given the job of moving a refrigerator of mass 100 kg across a horizontal floor. The coefficient of static friction between the refrigerator and the floor is 0.45. What is the minimum force...
-
Your moving company runs out of rope and hand trucks, so you are forced to push two crates along the floor as shown in Figure P3.39. The crates are moving at constant velocity, their masses are m 1 -...
-
x + y If x + 5=5+ y and x, y are positive integers, then the value of x + y is
-
The graph of y = g(x) is shown. Draw the graph of y = g (*). 6 -8 -6 -2 4. 6 -6-
-
An investment banker is analyzing two companies that specialize in the production and sale of candied yams. Cullumber Yams uses a labor-intensive approach, and Ivanhoe Yams uses a mechanized system....

Study smarter with the SolutionInn App


