Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction
Question:
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow.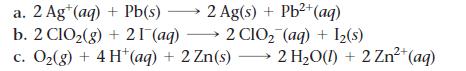
Transcribed Image Text:
a. 2 Ag+ (aq) + Pb(s) → 2 Ag(s) + Pb²+ (aq) b. 2 C1O₂(g) + 21 (aq) 2 CIO₂ (aq) + 1₂(s) c. O₂(g) + 4H+ (aq) + 2 Zn(s) → 2 H₂O(l) + 2 Zn²+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
4 Anode Pb Pb Pbs 2 Pb aq 2 e Salt bridge Ag A...View the full answer

Answered By

Sarfraz gull
have strong entrepreneurial and analytical skills which ensure quality tutoring and mentoring in your international business and management disciplines. Over last 3 years, I have expertise in the areas of Financial Planning, Business Management, Accounting, Finance, Corporate Finance, International Business, Human Resource Management, Entrepreneurship, Marketing, E-commerce, Social Media Marketing, and Supply Chain Management.
Over the years, I have been working as a business tutor and mentor for more than 3 years. Apart from tutoring online I have rich experience of working in multinational. I have worked on business management to project management.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the...
-
Use line notation to represent each electrochemical cell in Problem 44. Problem 44 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that...
-
Use line notation to represent each electrochemical cell in Problem 43. Problem 43 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that...
-
Determine whether the given functions are even, odd, or neither. a. x sin(x) c. x cos(x) e. x sin(x) + x sin(x) f. x sin(x) + x cos(x) even h. x sin(x) + x cos(x) j. x cos (x) + x cos(x) b. x sin(x)...
-
Garden Metal Works produces lawn sculptures. The company analyzes only variances that differ by more than 5 percent from the standard cost. The controller computed the following direct labor...
-
Hansen and Companys annual overhead budget equals $7,200,000 of fixed costs plus $80 per labor hour in variable costs. For the most recent year, budgeted direct labor hours were 100,000. Actual total...
-
Gorman Enterprises sells on account. When a customer account becomes four months old, Gorman converts the account to a note receivable. During 2010, Gorman completed these transactions: Requirement...
-
Coca-Cola is a well-established consumer products company with a strong position in the global market. The sales of their core soda products have remained relatively stable for decades, yet the...
-
K Dunn Manufacturing's variable costs are 40% of sales. The company is contemplating an advertising campaign that will cost $46,000. If sales are expected to increase $87,000, by how much will the...
-
Calculate the standard cell potential for each of the electrochemical cells in Problem 43. Problem 43 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the...
-
Balance each redox reaction occurring in basic aqueous solution. a. MnO4 (aq) + Br (aq) MnO(s) + BrO3(aq) b. Ag(s) + CN- (aq) + O(g) Ag(CN) (aq) c. NO (aq) + Al(s) - NH3(g) + AlO (aq)
-
A proton and a neutron interact via the strong nuclear force. Their interaction is mediated by a meson, much like the interaction between charged particles is mediated by photons-the particles ol the...
-
if x and y are integers and their values can be between 0 lesser than x lesser then 5 0 lesser than y lesser than 4 What are all the integer values for - 2x + y
-
What will be the output of the following Java code? 1. class operators public static void main(String args[]) 2. { 3. 4. { 5. 6. 7. 8. 9. 10. } 11. } int var1 = 5; int var2 6; int var3; = var3 = ++...
-
In game theory, a firm under "(non-corporative) simultaneous game" may make a strategic commitment so as to change the situation from simultaneous game to "sequential game" in which it plays a role...
-
Please use the financial statements for Youth Without Shelter (YWS) 2020-21 fiscal year. You can find them at the Youth Without Shelter website under Who We Are - Financials. YWS's operates an...
-
Problem 1: Your company is considering a project with an initial cash outlay of $500,000. The discount rate is 10%. Projected cash inflows for the next 5 years are: Year 1: $100,000; Year 2:...
-
You have the opportunity to invest $10,000 in one of two companies from a single industry. The only information you have is shown here. The word high refers to the top third of the industry; average...
-
1. Use these cost, revenue, and probability estimates along with the decision tree to identify the best decision strategy for Trendy's Pies. 2. Suppose that Trendy is concerned about her probability...
-
Consider a box with a lid 1.5 m wide and 0.70 m long. If the inside of the box is evacuated (i.e., its pressure is zero), how much force is required to open the lid? Could you open the lid?
-
Why does the lava in a Lavalamp (Fig. Q10.10) rise and then fall? Figure Q10.10
-
The pressure in the atmosphere is not constant, but fluctuates as the weather changes. If the pressure outside a window drops by 5% while the pressure inside does not change, what is the force on the...
-
The following items are reported on a company's balance sheet: Cash $296,600 Marketable securities 185,000 Accounts receivable (net) 121,000 Inventory 127,000 Accounts payable 228,000 Determine (a)...
-
Assume Major Link Inc. purchased a new piece of equipment on January 1, 2020, that cost $25,000. The estimated useful life is five years, and estimated residual value is $2,500. If Major Link uses...
-
Indicate the effect, if any, that each separate transaction has on financing cash flows. Note: Select "No Effect" if there is no effect. a. Long-term notes payable with a carrying value of $15,000...

Study smarter with the SolutionInn App


