The graphs labeled (a) and (b) show the titration curves for two equal-volume samples of bases, one
Question:
The graphs labeled (a) and (b) show the titration curves for two equal-volume samples of bases, one weak and one strong. Both titrations were carried out with the same concentration of strong acid.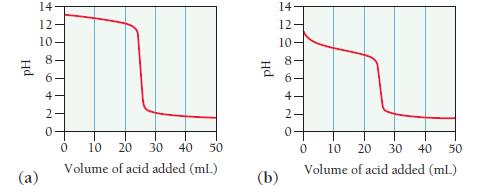
i. What is the approximate pH at the equivalence point of each curve?
ii. Which graph corresponds to the titration of the strong base and which one to the weak base?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





