The high-pressure phase diagram of ice is shown here. Notice that, under high pressure, ice can exist
Question:
The high-pressure phase diagram of ice is shown here. Notice that, under high pressure, ice can exist in several different solid forms. What three forms of ice are present at the triple point marked O? How does the density of ice II compare to ice I (the familiar form of ice)? Would ice III sink or float in liquid water?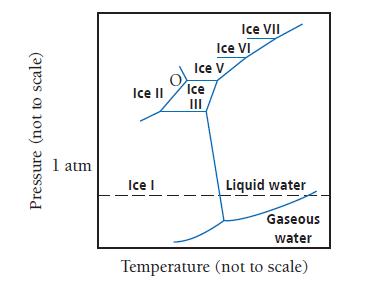
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





