The osmotic pressure of a solution containing 5.87 mg of an unknown protein per 10.0 mL of
Question:
The osmotic pressure of a solution containing 5.87 mg of an unknown protein per 10.0 mL of solution is 2.45 torr at 25 °C. Find the molar mass of the unknown protein.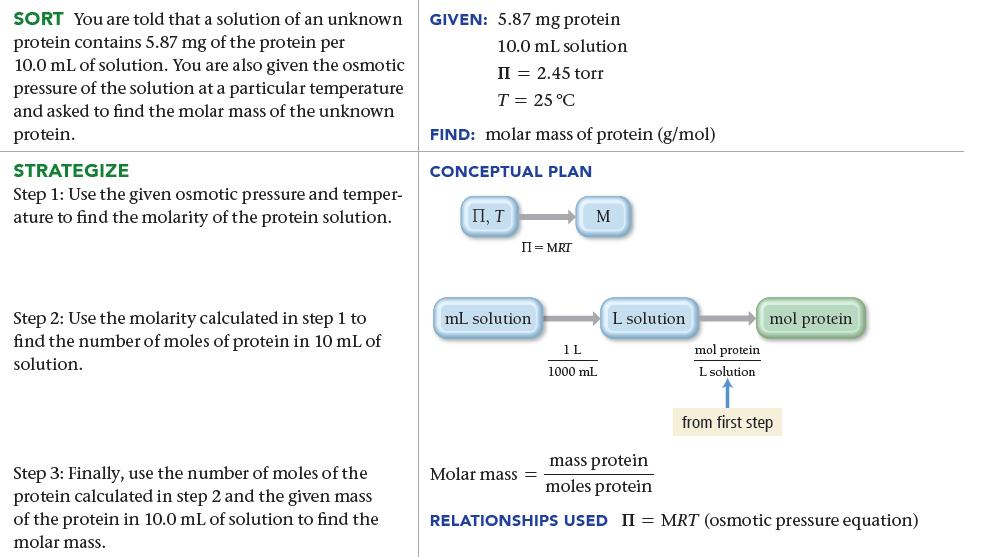
Transcribed Image Text:
SORT You are told that a solution of an unknown protein contains 5.87 mg of the protein per 10.0 mL of solution. You are also given the osmotic pressure of the solution at a particular temperature and asked to find the molar mass of the unknown protein. STRATEGIZE Step 1: Use the given osmotic pressure and temper- ature to find the molarity of the protein solution. Step 2: Use the molarity calculated in step 1 to find the number of moles of protein in 10 mL of solution. Step 3: Finally, use the number of moles of the protein calculated in step 2 and the given mass of the protein in 10.0 mL of solution to find the molar mass. GIVEN: 5.87 mg protein 10.0 mL solution II = 2.45 torr T = 25 °C FIND: molar mass of protein (g/mol) CONCEPTUAL PLAN П, Т II = MRT mL solution. M Molar mass= 1L 1000 mL L solution mol protein L solution mol protein from first step mass protein moles protein RELATIONSHIPS USED II = MRT (osmotic pressure equation)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
II MRT M II RT 245 terf X 100 mL X 008206 ...View the full answer

Answered By

Akash Goel
I am in the teaching field since 2008 when i was enrolled myself in chartered accountants course
Since then i have an experience of teaching of class XI, XII, BCOM, MCOM, MBA, CA CPT.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The osmotic pressure of a solution containing 22.7 mg of an unknown protein in 50.0 mL of solution is 2.88 mmHg at 25 C. Determine the molar mass of the protein. a) 246 g/mol b) 3.85 g/mol c) 2.93 *...
-
A solution containing 27.55 mg of an unknown protein per 25.0 mL solution was found to have an osmotic pressure of 3.22 torr at 25 C. What is the molar mass of the protein?
-
Calculate the osmotic pressure of a solution containing 18.75 mg of hemoglobin in 15.0 mL of solution at 25 C. The molar mass of hemoglobin is 6.5 * 10 4 g/mol.
-
A website that reviews recent movies lists 6 five-star films (the highest rating), 17 four-star films, 14 three-star films, 9 two-star films, and 4 one-star films. Make a frequency table for the data...
-
Nico Parts, Inc., produces electronic products with short life cycles (of less than two years). Development has to be rapid, and the profitability of the products is tied strongly to the ability to...
-
Show that the probability density function of a negative binomial random variable equals the probability density function of a geometric random variable when r _ 1. Show that the formulas for the...
-
What is a System Retirement/Disposal Concept? Identify examples of its UCs.
-
Adam Singh, D.D.S., opened an incorporated dental practice on January 1, 2012. During the first month of operations the following transactions occurred: 1. Performed services for patients who had...
-
As a member of the IT team atDon & Associates,a financial consulting company that provides services to small-and medium-sized companies. Because the company is looking to expandinto neighboring...
-
What is the heat of hydration ( Hhydration )? How does the enthalpy of solution depend on the relative magnitudes of Hsolute and Hhydration ?
-
Which of these aqueous solutions has the highest boiling point? a) 1.25 M C 6 H 12 O 6 b) 1.25 M KNO 3 c) 1.25 M Ca(NO 3 ) 2 d) None of the above (they all have the same boiling point)
-
Acme Company has a January 15 mid-month gross salaries expense of $25,000. All is subject to FICA Social Security (6.2%), FICA Medicare (1.45%), state income tax (5%) and federal income tax (15%)...
-
Yellow Sticker Company's variable expenses are 40% of sales. The company has monthly fixed expenses of $15,000 and sells each unit for $0.50. The monthly target operating income is $7,500. a. What is...
-
On June 1, Blue Spruce Corp. Ltd. borrows $76,500 from Acme Bank on a 6-month, $76,500, 8% note. The note matures on December 1. (a) Prepare the entry on June 1. (Credit account titles are...
-
Suppose a bank currently has $240,000 in deposits and $23,000 in reserves. The required reserve ratio is 13%. If at the end of the day, there is an unexpected withdrawal of $4,000 in reserves, what...
-
As the drawing shows, a carpenter on a space station has constructed a 30 deg ramp. A rocket moves past the space station with a relative speed of 0.884c in a direction parallel to side x. What does...
-
At a bowling alley, two players each score a spare when their bowling balls make head-on, approximately elastic collisions at the same speed with identical pins. After the collisions, the pin hit by...
-
Discuss uses of EPS and reasons or objectives for the current method of reporting EPS.
-
Suppose the index goes to 18 percent in year 5. What is the effective cost of the unrestricted ARM?
-
Stemming a chimney. A rock climber of mass 60 kg wants to make her way up the crack between two rocks as shown in Figure P4.14. The coefficient of friction between her shoes and the rock surface is...
-
Give an example in which the acceleration is perpendicular to the velocity.
-
For the system in Problem 12 and Figure P4.12, how large can m 2 be made without the system starting into motion? Data From Problem 12 Two blocks of mass m 1 = 45 kg and m 2 = 12 kg are connected by...
-
How do the monetization strategies of social networks, such as targeted advertising and user data exploitation, impact user experience and societal norms ?
-
what ways do social structures and institutional power dynamics perpetuate systemic inequalities across different societies, and how might these structures be effectively challenged or transformed to...
-
In each of the following examples, (i) draw the original supply and demand curve; (ii) illustrate graphically how changes described in the example would be reflected by a shift in the supply curve or...

Study smarter with the SolutionInn App


