The tabulated data were collected for the second-order reaction: Cl(g) + H 2 (g) HCl(g) +
Question:
The tabulated data were collected for the second-order reaction:
Cl(g) + H2(g) → HCl(g) + H(g)
Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction.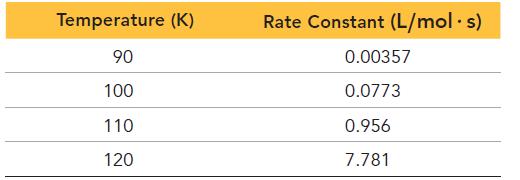
Transcribed Image Text:
Temperature (K) 90 100 110 120 Rate Constant (L/mol. s) 0.00357 0.0773 0.956 7.781
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Ea 23...View the full answer

Answered By

Amar Kumar Behera
I am an expert in science and technology. I provide dedicated guidance and help in understanding key concepts in various fields such as mechanical engineering, industrial engineering, electronics, computer science, physics and maths. I will help you clarify your doubts and explain ideas and concepts that are otherwise difficult to follow. I also provide proof reading services. I hold a number of degrees in engineering from top 10 universities of the US and Europe.
My experience spans 20 years in academia and industry. I have worked for top blue chip companies.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The tabulated data show the rate constant of a reaction measured at several different temperatures. Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction....
-
The tabulated data show the rate constant of a reaction measured at several different temperatures. Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction....
-
The following data show the rate constant of a reaction measuredat several different temperatures. Temperature (K) Rate Constant (1/s) 300 7.5610 - 2 310 0.221 320 0.605 330 1.56 340 3.79 Part A Use...
-
What would a profile look like across a restraining bend? Releasing bend?
-
Spencer Company manufactures and sells three products. Relevant per unit data concerning each product are given below. Instructions (a) Compute the contribution margin per unit of the limited...
-
Derek Lee just received a signing bonus of $1,000,000. His plan is to invest this payment in a fund that will earn 6%, compounded annually. Instructions (a) If Lee plans to establish the DL...
-
What performance measure would you consider most important for McDonald's? For Chevrolet?
-
Arsenal Electronics is going to construct a new $1.2 billion semiconductor plant and has selected four towns in the Midwest as potential sites. The important location factors and ratings for each...
-
Problem 7. [5 pts] Consider the scalar field f: R R such that f(x) = ||x||7. Compute the gradient of f. Vf(x) = Problem 8. [10 pts] Consider the scalar field : R \ {0} the gradient of f. Vf(x) = R...
-
A reaction has a rate constant of 0.000122/s at 27 C and 0.228/s at 77 C. a. Determine the activation barrier for the reaction. b. What is the value of the rate constant at 17 C?
-
The rate constant (k) for a reaction was measured as a function of temperature. A plot of ln k versus 1/T (in K) is linear and has a slope of -1.01 * 10 4 K. Calculate the activation energy for the...
-
Chopade and co-workers in 2003 reported the vaporliquid, liquidliquid, and vaporliquidliquid equilibrium for the system 2-methyl-1, 3 dioxolane (1) + water (2) system at 1 atm. They abbreviate the...
-
Name six commodity products which have forward and futures trades. Can you think of a commodity product not related to natural resources which has forward and futures trades?
-
More and more Western countries expatriation packages are disappearing and being replaced by local contracts. Why? What are the advantages and disadvantages?
-
Is digital remote working (working from anywhere) consolidating the globalization of enterprise? What is your answer If you are pro-globalization, and if you are anti-globalization?
-
Among the enterprises that you know, can you identify one that qualifies as a global company? Why?
-
What decision capabilities should the future HR department of Wells Fargo possess?
-
The following information pertains to Bell and Tower companies at the end of 2013: Required a. Compute each companys debt to assets ratio, current ratio, and times interest earned (EBIT must be...
-
Consider the function f and its graph. a. Estimate the zeros of the area function b. Estimate the points (if any) at which A has a local maximum or minimum. c. Sketch a graph of A, for 0 x 10,...
-
Find v(t) for t > 0 in the circuit in Fig. 16.68 . t = 0
-
For the circuit in Fig. 16.67 , find i(t) for t > 0. 10 2 Li(t) 10 mF 24u(t) A 120 V (+ 40 2 4 H ll
-
Determine i(t) for t > 0 in the circuit of Fig. 16.66 . t = 0 Li(t) 5 H3 2% F 36 V (+ 5
-
4. Write down the multiplication table for G when n is 16 and when n is 15.
-
Evaluating Exponential Functions. Evaluate each function at the indicated value. f ( x ) = - 5 ^ 3 x + 2 . f ( - 2 ) .
-
The ancient Greek mathematician Euclid is credited with the development of the theorem that the sum of the angles of a triangle is 180 degrees. Use the previous information to solve for the measure...

Study smarter with the SolutionInn App


