This reaction has an equilibrium constant of Kp = 2.2 * 10 6 at 298 K. Calculate
Question:
This reaction has an equilibrium constant of Kp = 2.2 * 106 at 298 K.![]()
Calculate Kp for each reaction and predict whether reactants or products will be favored at equilibrium.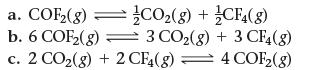
Transcribed Image Text:
2 COF2(g) = CO₂(g) + CF4(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
The given reaction is 2COF29 COg CF49 The equilibrium constant Kp for this reaction is 22 10 at 298 ...View the full answer

Answered By

Joan Gakii
I'm a meticulous professional writer with over five years writing experience. My skill set includes
- Digital Content,
- Interpersonal Communication,
- Web Content and academic Writing,
- Proofreading,
- Editing,
- Project Management, and
- Public Relations.
5.00+
7+ Reviews
12+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
This reaction has an equilibrium constant of K p = 2.26 * 10 4 at 298 K. Calculate K p for each reaction and predict whether reactants or products will be favored at equilibrium. CO(g) + 2 H(g) =...
-
A particular reaction has an equilibrium constant of K p = 0.50. A reaction mixture is prepared in which all the reactants and products are in their standard states. In which direction does the...
-
The reaction A2(g) + B(g) A(g) + AB(g) has an equilibrium constant of Kp = 2. The accompanying diagram shows a mixture containing A atoms (red), A2 molecules, and AB molecules (red and blue). How...
-
12. For a piece of material, the steady state flow of material across its thickness is 0.250 x 10 kg/m-hr. If the concentrationon the high side of the material is 1.85 kg/m and is 0.06 kg/m on the...
-
Each of the following scenarios is independent. All cash flows are after-tax cash flows. Required: 1. Jeffrey Akea has purchased a tractor for $62,500. He expects to receive a net cash flow of...
-
Answer the questions of Problem 12 for a point P for which the distance to the far speaker is 1 greater than the distance to the near speaker. Assume that the intensity at point P due to each speaker...
-
Jupiter's is considering an investment in time and administrative expense on an effort that promises one large payoff in the future, followed by additional expenses over a 10-year horizon. The cash...
-
Via Gelato is a popular neighborhood gelato shop. The company has provided the following data concerning its operations: While gelato is sold by the cone or cup, the shop measures its activity in...
-
If $300,000 is to be saved over 15 years, how much should be deposited monthly if the investment earns 7% interest compounded monthly?
-
Consider the reactions and their respective equilibrium constants: Use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction: NO(g) + Br(g) ...
-
A chemist trying to synthesize a particular compound attempts two different synthesis reactions. The equilibrium constants for the two reactions are 23.3 and 2.2 * 10 4 at room temperature. However,...
-
What is GE's current level of diversification? Historically, has GE been becoming more or less diversified? Why has GE been moving in this direction?
-
Why is there no optimal structure?
-
How do benchmarking and trend data differ?
-
What are the major substitutes for hospital care? How do these substitutes vary by hospital service?
-
In what situations could breakeven analysis be used effectively?
-
Does an organizations strategic intent always need to specify a mission, a vision, and values separately? Why or why not?
-
Based on predicted production of 24,000 units, a company anticipates $ 300,000 of fixed costs and $ 246,000 of variable costs. If the company actually produces 20,000 units, what are the flexible...
-
Access the Federation of Tax Administrators Internet site at www. taxadmin.org/state-tax-forms and indicate the titles of the following state tax forms and publications: a. Minnesota Form M-100 b....
-
Consider the circuit in Fig. 2.118 . Find the equivalent resistance at terminals: (a) a-b, (b) c-d. 450 2 10 2 300 2 300 2 450 2 60 2
-
For the circuit shown in Fig. 2.116 , find the equivalent resistance. All resistors are 3Ω. ww R. 'eq
-
Design a problem to help other students better understand wye-delta transformations using Fig. 2.114. What value of R in the circuit of Fig. 2.114 would cause the current source to deliver 800 mW to...
-
What are the three basic rights of employees in Canada ? Which employees have limited rights due to their job responsibilities? Give appropriate example
-
Analyze the concepts and theories used facilitate the change process and sustainability of the change effort
-
Briefly outline the key points of the case found in the Lexis-Nexis database

Study smarter with the SolutionInn App


