Two liquids, A and B, have vapor pressures at a given temperature of 24 mmHg and 36
Question:
Two liquids, A and B, have vapor pressures at a given temperature of 24 mmHg and 36 mmHg, respectively. We prepare solutions of A and B at a given temperature and measure the total pressures above the solutions. We obtain these data: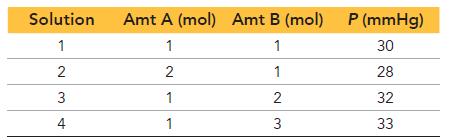
Predict the total pressure above a solution of 5 mol A and 1 mol B.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





