Under certain conditions, sodium reacts with oxygen to form sodium oxide according to the reaction: A flask
Question:
Under certain conditions, sodium reacts with oxygen to form sodium oxide according to the reaction:![]()
A flask contains the amount of oxygen represented by the diagram shown at far right.
Which of the following images best represents the amount of sodium required to completely react with all of the oxygen in the flask according to the equation?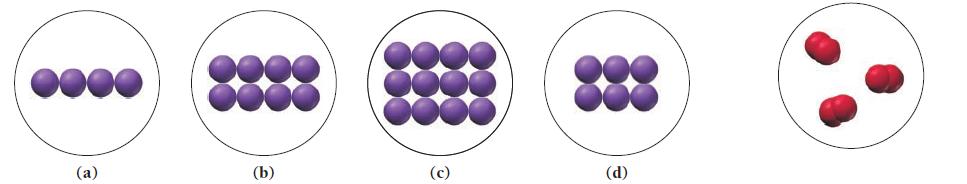
Transcribed Image Text:
4 Na(s) + O₂(g) 2 Na₂O(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
c Since each O 2 mol...View the full answer

Answered By

Mahesh G
I have more than 7 years of experience in teaching physics, mathematics and python programming to more than 600 students including both online and offline tutoring.
I follow the following 7 step fundamental approach towards tutoring.
1. Curiosity, scope, enlightenment of the topic in hand.
2. Problem Definitions and elaboration.
3. Requisite mathematics, analytical abilities and quantitative
aptitude.
4. Preparing Algorithms for problem statement.
5. Concepts with analogies and building algorithm.
6. Introspection and improvising.
7. Daily class wise Cheat sheets(its not cheating) for consolidation.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. Consider a Boeing 747 airliner cruising at a velocity of 550 mile/hr at a standard altitude of 38,000 ft, where the freestream pressure and temperature are 432.6 lb/ft and 390 R, respectively. A...
-
Nitrogen and hydrogen gas react to form ammonia according to the reaction: A flask contains a mixture of reactants represented by the image shown at the left. Which of the following images best...
-
A sample of 10.00 g of sodium reacts with oxygen to form 13.83 g of sodium oxide (Na2O) and sodium peroxide (Na2O2). Calculate the percent composition of the mixture.
-
Delmott sells a snowboard, Xpert that is popular with snowboard enthusiasts. Below is information relating to Delmotts purchases of Xpert snowboards during September. During the same month, 102 Xpert...
-
The income statement of Belini Inc. reported the following condensed information. Belinis balance sheet contained these comparative data at December 31. Belini has no depreciable assets. Accounts...
-
A garment soaked with water is hung up to dry in a warm room at atmospheric pressure. The still air is dry and at a temperature of 40C. The garment may be assumed to have a temperature of 25C and a...
-
Outline the general strategy used in metagenomics.
-
The Holtz Corporation acquired 80 percent of the 100,000 outstanding voting shares of Devine, Inc., for $7.20 per share on January 1, 2014. The remaining 20 percent of Devines shares also traded...
-
Consider the HMM where the underlying Markov chain is given by the state transition diagram below. The observations are such that the true state is observed 50% of the time and each other state is...
-
The partnership of Par and Boo was formed and commenced operations on March 1, 2016, with Par contributing $30,000 cash and Boo investing cash of $10,000 and equipment with an agreed-upon valuation...
-
What is a balanced chemical equation?
-
During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction: Suppose that a particular plant consumes 37.8 g of CO 2 in one week. Assuming...
-
A U.S.-based MNC has a subsidiary in France (local currency, euro, ;). The balance sheet and income statement of the subsidiary follow. On December 31, 2112, the exchange rate is US$1.20/;. Assume...
-
The project's outcome is focused management and supervision of improvements in the surgical department by nurse managers. How can the Christian World View (CWV) impact the project, if at all? Does...
-
Complete the self-assessment online quiz: Moment of Truth: Online Ethics Quiz Links to an external site.(Media Partners, 2018)Links to an external site. Informed by the Quiz results, outline a plan...
-
Microtubules are filamentous structures in cells that maintain cell shape and facilitate the movement of molecules within the cell. They are long, hollow cylinders with a diameter of about 25 nm. It...
-
The group you are working with have come to a stand-still due to a problem that seems impossible to resolve. Members are starting to show frustration and despite much talk they are no closer to...
-
Consider the following program. For each of the following parameter-passing methods, what is printed? a. Passed by value b. C. void main() Passed by reference Passed by value-result { int x = 5; foo...
-
Identify the errors in the following schedule of cost of merchandise sold for the current year ended May 31,2013: Cost of merchandise sold: 155,000 Merchandise inventory, May 31, 2013 Purchases Plus:...
-
Havel says the grocer doesnt believe what is on the sign and indeed, he says the grocers customers will barely notice it. But Havel maintains that the sign serves a specific function. How would you...
-
In Equation (8.16), (dP/dT) vaporization was calculated by assuming that V gas m >> V liquid m . In this problem, you will test the validity of this approximation. For water at its normal boiling...
-
The densities of a given solid and liquid of molar mass 122.5 gmol 1 at its normal melting temperature of 427.15 K are 1075 and 1012 kgm 3 , respectively. If the pressure is increased to 120. bar,...
-
The variation of the vapor pressure of the liquid and solid forms of a pure substance near the triple point are given by ln P solid /Pa = -8750K/T + 34.143 and In P liquid /Pa = -4053K/T + 21.10....
-
The figure depicts Jack - in - the - box: "Jack" is attached inside a box by a spring, as shown. You estimate Jack's mass to be 0 . 4 k g . As so often happens, a sign tells you the spring constant:...
-
Keynesians believe that the tools (G, T and MS) should be used to try to help the economy. Should we? Why or why not?
-
How might actions/events in other countries outside the U.S. effect the aggregate supply or aggregate demand in the U.S.? Be sure to explain.

Study smarter with the SolutionInn App


