Use data from Appendix IIB to calculate S rxn for each of the reactions. In each case,
Question:
Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn.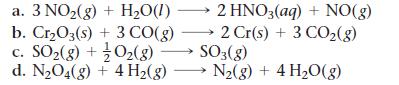
Appendix IIB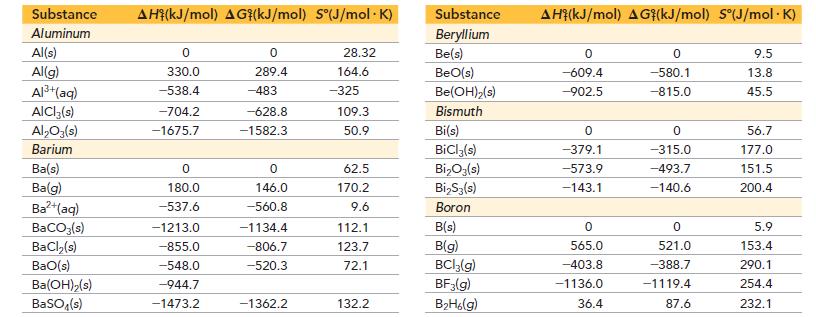
Transcribed Image Text:
a. 3 NO₂(g) + H₂O(1)→ b. Cr₂O3(s) + 3 CO(g) c. SO₂(g) + O₂(g) 2 HNO3(aq) + NO(g) 2 Cr(s) + 3 CO₂(g) SO3(g) d. N₂O4(g) + 4H₂(g) →→→ N₂(g) + 4H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 3NO2g H2Ol 2HNO3aq NOg ASPx 2 SHNO3aq 1 SOg 3 SNOg 1 SO1 Substitute the values ...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use data from Appendix IIB to calculate S rxn for each of the reactions. In each case, try to rationalize the sign of S rxn . Appendix IIB a. CH4(g) + H(g) CH6(g) b. C(s) + HO(g) CO(g) + H(g) c....
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. G f for BrCl(g) is -1.0 kJ/mol. Appendix IIB a. 2 NO(g) NO4(8) b. Br(g) + Cl(g) = 2 BrCI(g)
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. Appendix IIB a. 2 CO(g) + O(g) = 2 CO(g) b. 2 HS(g) = 2 H(g) + S(g)
-
Q1-Mutual funds provide the following for their shareholders. A. diversification B. professional management C. record keeping and administration D. all of these options
-
Discuss the strategic implications of marketing in Mexico.
-
Assume Ashland Community Hospital had the following supplies costs for two products used in its operating room. Standard costs for one surgery: Item X, 5 pieces at $50 each; Item Y, 10 pieces at $75...
-
Tennessee law imposes durational-residency requirements on persons and companies wishing to operate retail liquor stores, requiring applicants for an initial license to have resided in the state for...
-
A company manufactures three products: A, B, and C. The company currently has an order for three units of product A, 7 units of product B, and 4 units of product C. There is no inventory for any of...
-
The following are the ages of 13 mathematics teachers in a school district. 28, 30, 34, 34, 36, 38, 39, 42, 46, 47, 49, 50, 51 Notice that the ages are ordered from least to greatest. Give the...
-
Find S for the formation of CH 2 Cl 2 ( g) from its gaseous elements in their standard states. Rationalize the sign of S.
-
Rank each set of substances in order of increasing standard molar entropy (S). Explain your reasoning. a. 1(g); F(g); Br2(g); Cl(g) b. HO(g); HO(g); HS(g) c. C(s, graphite); C(s, diamond); C(s,...
-
Consider the data in Exercise 15-45. Use the Wilcox on signed-rank test for this problem with a = 0.05. What conclusions can you draw? Does the hypothesis you are testing now differ from the one...
-
Consider the function f(x) = 12-x3-27 x + 4x +8. Find the antiderivative of f(x).
-
What do you think about user experience? This area of digital communications falls more under design, but how your site navigation and structure work are definitely forms of communication. I'd love...
-
How are you trying to envision your personal future in uncertain times? To what extent are you relying on media to help you assess job prospects in various careers? Are media helping you decide what...
-
1. Why may it be preferable to buy from a distributor or wholesaler rather than directly from manufacturer? 2. How could social or political issues impact a supplier selection decision? 3. What are...
-
What should you do if you notice workplace procedures may not comply with legislation in regards to hazards and risks?
-
The economic analysis carried out during project identification and selection is rather superficial. Why is this? Consequently, what factors do you think tend to be most important for a potential...
-
In 1995 Miguel purchased a home for $130,000. In 2000 he sold it for $170,000 and immediately purchased another one for $180,000, which he sold in 2007 for $235,000. How much taxable capital gain, if...
-
Consider two cylinders with the same density and the same length. If the ratio of their diameters is 1.5/1, what is the ratio of their moments of inertia?
-
In our examples with CDs (such as Example 8.7), we usually ignored the hole in the center. Calculate the moment of inertia of a CD, including the effect of the hole. For a CD of radius 6.0 cm,...
-
The moment of inertia for a square plate of mass M and length L that rotates about an axis perpendicular to the plane of the plate and passing through its center is ML 2 /6 (Fig. P8.49A). What is the...
-
An investor buys a 10-year bond with a 6.5% annual coupon and a YTM of 6%. Before the first coupon payment is made, the YTM for the bond decreases to 5.5%. Assuming coupon payments are reinvested at...
-
What's the price of a $50 par value 7% preferred share if the required rate of return is 8%?
-
Let's imagine a block on a sheet of ice, with negligible friction. The block has a mass of 5.00 kg. The block is currently moving to the right at 4.00 m/s. Let's say a rope is attached to this block,...

Study smarter with the SolutionInn App


