Use data from Appendix IIB to calculate S rxn for each of the reactions. In each case,
Question:
Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn.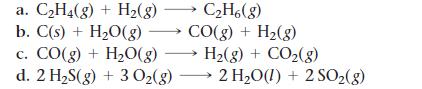
Appendix IIB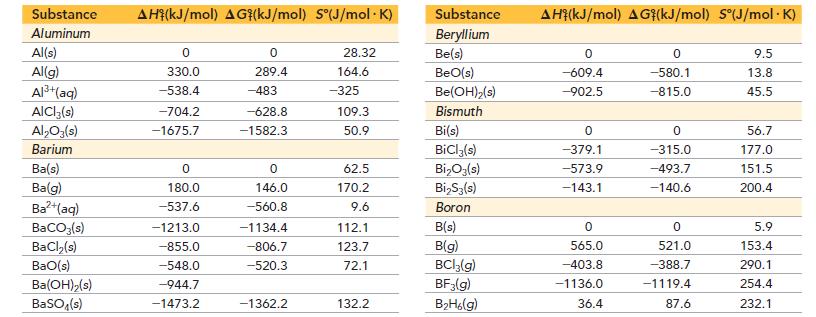
Transcribed Image Text:
a. C₂H4(g) + H₂(g) →→→ C₂H6(g) b. C(s) + H₂O(g) →→→ CO(g) + H₂(g) c. CO(g) + H₂O(g) → H₂(g) + CO₂(g) d. 2 H₂S(g) + 3 O₂(g) → 2 H₂O(l) + 2 SO₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
a 1208 JK decrease in moles of g...View the full answer

Answered By

Kennedy Odhiambo
As a professional writer, I have been in the field for over 5 years having worked as a lecture in different tertiary institutions across the world. With this impeccable experience, I assure provision of a good and supporting environment for students to learn.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use data from Appendix IIB to calculate S rxn for each of the reactions. In each case, try to rationalize the sign of S rx n . Appendix IIB a. 3 NO(g) + HO(1) b. CrO3(s) + 3 CO(g) c. SO(g) + O(g) 2...
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. G f for BrCl(g) is -1.0 kJ/mol. Appendix IIB a. 2 NO(g) NO4(8) b. Br(g) + Cl(g) = 2 BrCI(g)
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. Appendix IIB a. 2 CO(g) + O(g) = 2 CO(g) b. 2 HS(g) = 2 H(g) + S(g)
-
Recession is defined as... a) increase in unemployment b) decrease in consumer spending c) two consecutive quarters of negative economic growth d) both A & B
-
One of the ramifications of emerging markets is the creation of a middle class. Discuss.
-
College Coffee Shops operates coffeehouses on college campuses in three districts. The operating performance for each district follows. a. Using the operating profit margin percentage as the...
-
According to the Supreme Court decision in Quill Corp. v. North Dakota, a state may not require a retailer with no physical presence in the state to collect and remit sales tax on sales made in the...
-
Following are the auditor's calculations of several key ratios for Cragston Star Products. The primary purpose of this information is to understand the client's business and assess the risk of...
-
1/ You have two different assets (investments). Asset A (perpetuity) will pay you $1,000 in one year, $1,000 in two years, $1,000 in three years, and so on every year forever. Asset B will pay you...
-
Rank each set of substances in order of increasing standard molar entropy (S). Explain your reasoning. a. 1(g); F(g); Br2(g); Cl(g) b. HO(g); HO(g); HS(g) c. C(s, graphite); C(s, diamond); C(s,...
-
Rank each set of substances in order of increasing standard molar entropy (S). Explain your reasoning. a. NH3(g); Ne(g); SO(g); CH3CHOH(g); He(g) b. HO(s); HO(1); HO(g) C. CH4(g); CF4(g); CC14(g)
-
Refer to the study of patients with substantial blockage of the arteries presented at the 2007 Annual Conference of the American College of Cardiology, Exercise 8.56. Recall that half the patients...
-
How has digital media affected your relationships? Does it enhance or inhibit connections with others?
-
Give informative speech to a group of new students concerning adapting to college life and responsibilities. discuss 3 organizational patterns you think would be appropriate and 3 points you could...
-
Explain adjustment messages that salvage customers' trust and promote further business with Revealing good news Up Front, Explaining Compliance in the Message Body, Deciding Whether to apologize,...
-
Described why each of us believes what we do; Explain why we choose to respond in a particular way when faced with an ethical dilemma; Define how you will respond when confronted with an ethical...
-
How do I use my relationship-building skills to express to my supervisor how I feel about delivering an impromptu speech without notes and a plan?
-
One of the families at your center does not believe they have the time, abilities, skills or interests to help their children. They lack confidence in their child-rearing roles. How does your program...
-
How is use of the word consistent helpful in fraud reports?
-
A ceiling fan of radius 45 cm runs at 90 rev/min. How far does the tip of the fan blade travel in 1 hour?
-
Construct a plot of the angular displacement of a CD as a function of time. Use information from this chapter to attach quantitative labels (numbers and units) to your graph. Take t = 0 to be the...
-
A potters wheel of radius 0.20 m is turned on at t = 0 and accelerates uniformly. After 60 s, the wheel has an angular velocity of 2.0 rad/s. Find the angular acceleration and the total angular...
-
The isotope of plutonium 238. Pu. Part: 0 / 2 Part 1 of 2 238, Pu is used to make thermoelectric power sources for spacecraft. Suppose that a space probe was launched in 2012 with 2.5 kg of 238. (a)...
-
discuss the integration of meta-learning techniques, such as model selection or hyperparameter optimization, within the data mining pipeline to automate the process of algorithm selection and...
-
Discuss the tool and inventor who helped air crash investigators solve the mysteries of various aircraft accidents? Is the tool perfect? Define density altitude and describe the three factors that...

Study smarter with the SolutionInn App


