Use standard free energies of formation to calculate G at 25 C for each reaction in Problem
Question:
Use standard free energies of formation to calculate ΔG° at 25 °C for each reaction in Problem 62. How well do the values of ΔG° calculated this way compare to those calculated from ΔH° and ΔS°? Which of the two methods could be used to determine how ΔG° changes with temperature?
Problem 62
For each reaction, calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C and state whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 °C?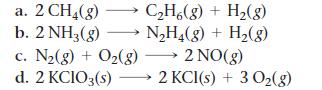
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





