Use Table 20.1 to determine which metal does not dissolve in hydrochloric acid (HCl). a) Zn b)
Question:
Use Table 20.1 to determine which metal does not dissolve in hydrochloric acid (HCl).
a) Zn
b) Cd
c) Cu
d) Fe
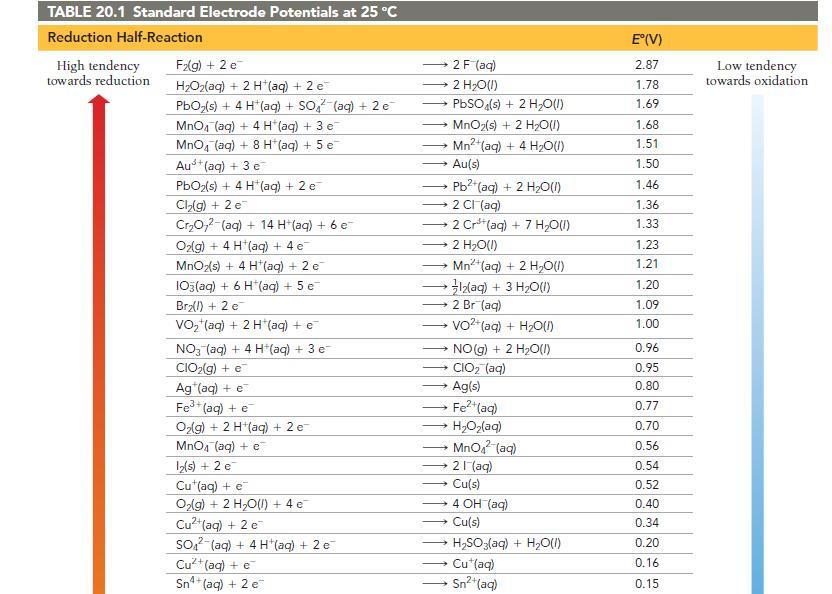
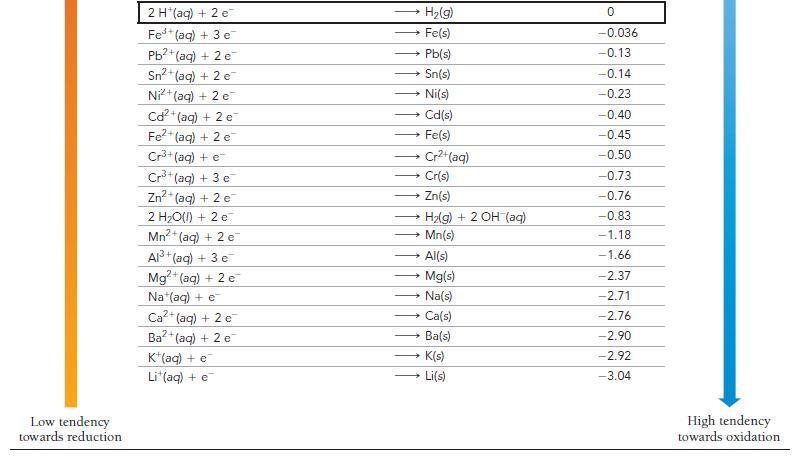
Transcribed Image Text:
TABLE 20.1 Standard Electrode Potentials at 25 °C Reduction Half-Reaction High tendency towards reduction F2(g) + 2 e H₂O₂(aq) + 2 H+ (aq) + 2 e PbO₂(s) + 4 H (aq) + SO MnO4 (aq) + 4 H (aq) + 3 e MnO4 (aq) + 8 H(aq) + 5 e Au³+ (aq) + 3 e PbO₂(s) + 4 H*(aq) + 2 e Cl₂(g) + 2 e Cr₂O72-(aq) + 14 H+ (aq) + 6 e- O2(g) + 4 H (aq) + 4 e MnO₂(s) + 4 H+ (aq) + 2 e 1O3(aq) + 6 H*(aq) + 5 e Br₂(l) + 2 e VO₂ (aq) + 2 H*(aq) + e NO3(aq) + 4 H (aq) + 3 e CIO₂(g) + e Ag (aq) + e Fe³+ (aq) + e O₂(g) + 2 H(aq) + 2 e MnO4 (aq) + e 12(s) + 2 e Cu (aq) + e O₂(g) + 2 H₂O(l) + 4e¯ Cu²+ (aq) + 2 e SO² (aq) + 4 H(aq) + 2 e Cu²+ (aq) + e Sn4+ 4+ (aq) + 2 e (aq) + 2 e - 2 F (aq) 2 H₂O(l) PbSO4(s) + 2 H₂O(1) MnO₂(s) + 2 H₂O(l) Mn²+ (aq) + 4H₂O(1) Au(s) Pb²+ (aq) + 2 H₂O(l) 2 CI (aq) 2 Cr³+ (aq) + 7 H₂O(1) 2 H₂O(l) Mn+ (aq) + 2 H₂O(l) • 3lz(aq) + 3 HjO(l) 2 Br (aq) VO²+ (aq) + H₂O(l) NO(g) + 2 H₂O(1) CIO₂ (aq) Ag(s) Fe²+ (aq) HyOzlaq) MnO² (aq) 21 (aq) Cu(s) 4 OH (aq) → Cu(s) H₂SO3(aq) + H₂O(1) Cu*(aq) Sn²+ (aq) E°(V) 2.87 1.78 1.69 1.68 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.96 0.95 0.80 0.77 0.70 0.56 0.54 0.52 0.40 0.34 0.20 0.16 0.15 Low tendency towards oxidation
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Kalyan M. Ranwa
I have more than seven years of teaching experience in physics and mechanical engineering.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Cu + reacts with neocuproine to form the colored complex (neocuproine)2Cu + , with an absorption maximum at 454 nm. Neocuproine is particularly useful because it reacts with few other metals. The...
-
Lanthanum metal forms cations with a charge of 3+. Consider the following observations about the chemistry of lanthanum: When lanthanum metal is exposed to air, a white solid (compound A) is formed...
-
Which of the following insoluble calcium compounds does not dissolve in hydrochloric acid? (a) Limestone, CaCO 3 (b) Slaked lime, Ca(OH) 2 (c) Gypsum, CaSO 4 2 H 2 O (d) Hydroxyapatite, Ca 5 (OH)(PO...
-
characterize the duplicate constructor utilized in c++ alongside its overall capacity model explaon the different situations which it is called what is the distinction between CSMA/CD/CSMA/CA what...
-
Roofing tile is the major product of the Tops Corporation. The company had a particularly good year, as shown by its operating data. It sold 88,400 cases of tile. Variable cost of goods sold was...
-
Thomson Media is considering some new equipment whose data are shown below. The equipment has a 3-year tax life and would be fully depreciated by the straight-line method over 3 years, but it would...
-
Using the variance data for Menounos Manufacturing Co., prepare an income statement through gross profit for the year ended December 31, 20Y6. Assume the company sold 3,000 units at $100 per unit.
-
Ready-Set-Go Co. distributes suitcases to retail stores and extends credit terms of 1/10, n/30 to all of its customers. At the end of June, Ready-Set-Go's inventory consisted of suitcases costing...
-
The index of refraction for a particular material is 1.38. Determine the speed of light in this medium. Light passes from water (n=1.33) into air (n=1.003) at an angle of incidence of 25 degrees....
-
Which metal dissolves in HNO 3 but not in HCl? (a) Fe (b) Au (c) Ag
-
Use tabulated standard electrode potentials to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25 C. (The equation is balanced.) Al(s) +...
-
Use the ending balances from Problem 10- 27 to prepare a balance sheet.
-
"What is the current understanding of the intricate crosstalk between the gut microbiome and the host immune system, and how do these interactions influence both local and systemic immunity, with...
-
Determine k for which vector = (5,-1, k) is t to v = (k+2, -3,2)
-
Why might an heir be happier to be the death beneficiary of a $5,000,000 life insurance policy held by an Irrevocable Life Insurance Trust, instead of being named to receive shares of stock valued at...
-
What is the molarity of a 350 ml solution, containing 13.6g AgNO3? (MM of AgNO3 is 169.87 g/mol)
-
Write 4-5 Pages Explain your self-assessment in relation to the nurse leadership competencies. Be sure to identify your strengths and potential gaps in relation to these competencies. Describe at...
-
Fixed rate notes and bonds have interesting dynamics that respond to various economic factors affecting the market or fair value of those instruments. There are several potential results from the...
-
1. Using the information from Problem 16-4B, prepare a statement of cash flows for Lim Garden Supplies Inc. using the direct method of presenting cash flows from operating activities. 2. How does Lim...
-
A bullet of mass m b = 25 g is fired with a speed of 250 m/s at a target that is a sheet of metal (mass m t = 500 g). The target is square with sides of length L = 20 cm. The target is hinged along...
-
A mass m 1 is connected to a pulley of radius R and mass m 2 as shown in Figure P9.74. The mass m 1 starts from rest and then falls through a distance h.? (a) Use conservation of energy to calculate...
-
Estimate the moment of inertia of a basketball? Table 8.2 (page 264) may be helpful. ? TABLE 8.2 Moment of Inertla for Some Common Objects Object Shape Object Shape Rod pivoted at center Hoop T = mR?...
-
This discussion board is open for any questions or comments in a new thread, but please address the following 1. Should we allow business to be conducted with limited liability entities? This...
-
You are taking an eye exam. You stand 5 m from a board that has letters printed on it. The separation between two of the letters on the board is 1 cm. Assume that the light in the room has a...
-
I think that if you are going to form a relationship (alliance) with anyone/company/etc. that you should comprehend each other's cultures, values, styles, etc. before joining forces. If they don't...

Study smarter with the SolutionInn App


