We can obtain titanium metal from its oxide according to the following balanced equation: When 28.6 kg
Question:
We can obtain titanium metal from its oxide according to the following balanced equation:![]()
When 28.6 kg of C reacts with 88.2 kg of TiO2, 42.8 kg of Ti is produced. Find the limiting reactant, theoretical yield (in kg), and percent yield.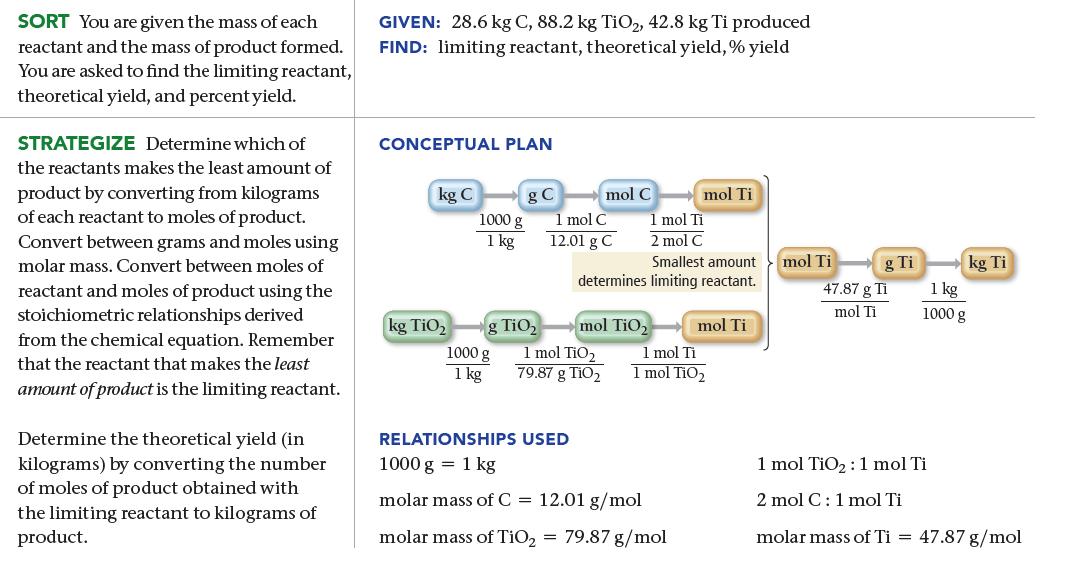
Transcribed Image Text:
TiO₂ (s) + 2 C(s) Ti(s) + 2 CO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
286 kg ex Limiting reactant 882 kgFiO X 1000g 1 kg 1000 X 1 kg 1 mot C ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The main reaction of a charcoal grill is C(s) + O2(g) CO2(g). Which of the statements below are incorrect? Why? a. 1 atom of carbon reacts with 1 molecule of oxygen to produce 1 molecule of CO2. b. 1...
-
Twelve years ago, Birch Ltd. (BL) borrowed $480,000 from Oak Trust Inc. (OTI). The 12- year, 10% note is due on todays date, December 31, 2020. The note was originally issued at par. BL is unable to...
-
What are some techniques of good writers? Which ones do you use regularly?
-
Rick Darman, the owner-president of Computer Services, is unfamiliar with the statement of cash flows that you, as his accountant, prepared. He asks for further explanation. Instructions Write him a...
-
Consider a horizontal pin fin of 6-mm diameter and 60-mm length fabricated from plain carbon steel (k = 57 W/m K, 8 = 0.5). The base of the fin is maintained at 150C, while the quiescent ambient air...
-
In 1951, DuPont began using the chemical perfluorooctanoic acid to manufacture Teflon. Due to the dangerous nature of the chemical, DuPont was given special instructions by its supplier to dispose of...
-
On January 5, 2012, Phelps Corporation received a charter granting the right to issue 5,000 shares of $100 par value, 8% cumulative and nonparticipating preferred stock, and 50,000 shares of $10 par...
-
What is the output of this code? x=3 num = 17 print(num %x)
-
Curtiss Construction Company, Incorporated, entered into a fixed-price contract with Axelrod Associates on July 1, 2024, to construct a four-story office building. At that time, Curtiss estimated...
-
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: Suppose that 5 mol NO 2 and 1 mol H 2 O combine and react completely. How many moles of the...
-
Sulfur and fluorine react to form sulfur hexafluoride: If 50.0 g S is allowed to react as completely as possible with 105.0 g F 2 , what mass of the excess reactant is left? a) 20.5 g S b) 45.7 g F 2...
-
Find the inverse of the function that is one-to-one. {(1, -3), (2, -7), (4, -3), (5, -5)}
-
The HIM manager recently performed an audit of the integrity of the master patient index. Her audit revealed of 200 patient records reviewed, 5% of those patients had a duplicate record created....
-
A company buys 7 new photocopying machines. Each machine costs $4,619. What is the total cost of the machines? Show your calculations.
-
Explain how clinical guidelines, clinical pathways and protocols can help in improving utilization management in healthcare? Provide examples of applying guidelines in the context of utilization...
-
< 1. Describe at least three types of tangible and intangible resources and capabilities. 2. In the text, are human resources used as an example of tangible or intangible resources? Do you agree with...
-
1. Describe divisional organizational structure and critically analyse the advantages and disadvantages of disadvantages of divisionalisation (use academic papers). 2. Bounce Ltd is a leading...
-
Illustrate the effects on the accounts and financial statements of the following related transactions of Seaside Diagnostic Company: a. Purchased $48,000 of merchandise from Davies Co. on account,...
-
The time to assemble the first unit on a production line is 10 hours. The learning rate is 0.94. Approximately how long will it take for the seventh unit to be assembled? The number of hours needed...
-
Calculate the van der Waals parameters of carbon dioxide from the values of the critical constants and compare your results with the values for and b in Table 7.4. Table 7.4 RedlichKwong van der...
-
Calculate the RedlichKwong parameters of fluorine from the values of the critical constants and compare your results with the values for a and b in Table 7.4. Table 7.4 RedlichKwong van der Waals b...
-
Calculate the critical volume for ethane using the data for T c and P c in Table 7.2 (see Appendix B, Data Tables) assuming a.The ideal gas equation of state b. The van der Waals equation of state....
-
Make relational model from this ER diagram 2. Convert the following ER diagram into relations/tables: row seat toCust SSNo Bookings Customers phone name Flights to Flt addr number aircraft day
-
First Draw an ER Model please and then covert it into relational model seperate figures required thank you Each cinema is identified by its name and has its residency at an address which consists of...
-
Transform the E-R model of project2 into a relational model. TransactionNo Date Hired Education Employee Address NumberOfItems Email 100 MemberD Name Transaction D Member TypeName Member Classify...

Study smarter with the SolutionInn App


