One mechanism for the destruction of ozone in the upper atmosphere is a. Which species is a
Question:
One mechanism for the destruction of ozone in the upper atmosphere is 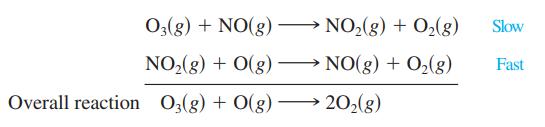
a. Which species is a catalyst?
b. Which species is an intermediate?
c. Ea for the uncatalyzed reaction
![]()
Is 14.0 kJ. Ea for the same reaction when catalyzed is 11.9 kJ. What is the ratio of the rate constant for the catalyzed reaction to that for the uncatalyzed reaction at 25°C? Assume that the frequency factor A is the same for each reaction.
Transcribed Image Text:
03(g) + NO(g). → NO₂(g) + O₂(g) NO₂(g) + O(g) →→→ NO(g) + O₂(g) Overall reaction O3(g) + O(g) 20₂(g) Slow Fast
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a NO is a catalyst A catalyst is a substance that increases the rate of a chemical reaction without ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
One pathway for the destruction of ozone in the upper atmosphere is O3(g) + NO(g) NO2(g) + O2(g) Slow NO2(g) + O(g) NO(g) + O2(g) Fast Overall reaction: O3(g) + O(g) 2O2(g) a. Which species is a...
-
A proposed two-step mechanism for the destruction of ozone in the upper atmosphere is a. What is the overall balanced equation for the ozone destruction reaction? b. Which species is a catalyst? c....
-
The rate of the reaction O(g) + NO2(g) NO(g) + O2(g) was studied at a certain temperature. This reaction is one step of the nitric oxide catalyzed destruction of ozone in the upper atmosphere. a. In...
-
Ashby and Curtis, married professionals, have a 2-year-old son, Jason. Curtis works full-time as an electrical engineer, but Ashby has not worked outside the home since Jason was born. As Jason is...
-
In October 1993, Marilyn Greenen, a licensed certified public accountant (CPA),began working at the Port of Vancouver, Washington (the Port), as an account manager. She was not directly engaged in...
-
A particle with charge 2.15µC and mass 3.20 X 10-11 kg is initially travelling in the +y-direc1ion with a speed U0 = 1.45 X 105 m/s. It then enters a region containing a uniform magnetic field...
-
An engineer prepares a report to evaluate a project using PW and IRR. Just before submitting the report, he spills coffee on it, making the first digit of the 2-digit IRR unreadable. The second digit...
-
VIP Corporation engaged in the transactions that follow. Identify each transaction as (a) An operating activity, (b) An investing activity, (c) A financing activity, (d) A noncash transaction, (e)...
-
Let M, N be closed subspaces of a Hilbert space H and P, Q the orthogonal projections with ran P = M, ran Q = N. Prove that the following conditions are equivalent:
-
Emerald City Umbrellas sells umbrellas and rain gear in Seattle, so its sales are fairly level across the year. However, it is branching out to other markets where it expects demand to be much more...
-
A first-order reaction has rate constants of 4.6 10 -2 s -1 and 8.1 10 -2 s -1 at 0C and 20.C, respectively. What is the value of the activation energy?
-
Which of the following conditions indicate a basic solution at 25C? a. pOH = 11.21 b. pH = 9.42 c. [OH-]> [H+] d. [OH-]> 1.0 10-7 M
-
Drake Company reported the following for 2017: Current assets............................................$87,000 Current liabilities..........................................19,000...
-
Antiqua owns a manufacturing facility. The annual gross receipts for the business were $25,750,000for tax year 2020. Based on the annual gross receipts, the business is no longer required to...
-
The maintenance expenses budget for next year will be based on the previous year's actual maintenance expenses of $373,000. The assumption is that these expenses will decrease by 7%. The budgeted...
-
B 9 1 2 3 4 5 6 7 Question 16 Input Area: Stock price at expiration Stock price at expiration 559 $ 53.00 60.00 Holding period (months) Strike price Output Area: At a stock price of Payoff per share...
-
What is an example of a financial statement from an agency in Texas ((not a private entity- public agencies only) that is most helpful in determining the fiscal health of that agency, and why?
-
Auditors use both statistical and nonstatistical sampling methods when selecting test transactions. In your own wrds, explain when you would use each method and describe the impact of the significant...
-
The Seago Company is planning to purchase $500,000 of equipment with an estimated seven-year life and no estimated salvage value . The company has projected the following annual cash flows for the...
-
When is the indirect pattern appropriate, and what are the benefits of using it?
-
Benzphetamine is an appetite suppressant that is marketed under the trade name Didrex and used in the treatment of obesity. Identify at least two different ways to make benzphetamine via a reductive...
-
Draw the product formed when each of the following compounds is treated with NaNO 2 and HCl: (a) (b) NH2 N.
-
Consider the structure of the azo dye called alizarine yellow R (below). Show the reagents you would use to prepare this compound via an azo coupling process. .N. N' O2N
-
Can you explain the necessary piece of information that would add value to understanding the employee's feeling and knowledged and capabilities of Amazon. Please cite sources and date
-
Production of boiler chickens (those raised for their meat, not eggs) in the US has grown from just over 10 billion in 1970 to nearly 60 billion pounds in 2021. Additionally, the average chicken...
-
"Animal spirits"optimism about and predictions for the current and future state of marketscan fuel increased spending on things like homes and financial instruments, even when those "spirits" are not...

Study smarter with the SolutionInn App


