A compound has been isolated that can have either of two possible formulas: (a) K[Fe(C 2 O
Question:
A compound has been isolated that can have either of two possible formulas:
(a) K[Fe(C2O4)2(H2O)2] or
(b) K3[Fe(C2O4)3]. To find which is correct, you dissolve a weighed sample of the compound in acid, forming oxalic acid, H2C2O4. You then titrate this acid with potassium permanganate, KMnO4 (the source of the MnO4− ion). The balanced, net ionic equation for the titration is
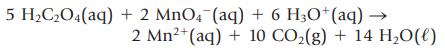 Titration of 1.356 g of the compound requires 34.50 mL of 0.108 M KMnO4. Which is the correct formula of the iron-containing compound: (a) or (b)?
Titration of 1.356 g of the compound requires 34.50 mL of 0.108 M KMnO4. Which is the correct formula of the iron-containing compound: (a) or (b)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





