Chromium(III) chloride forms many compounds with ammonia. To find the formula of one of these compounds, you
Question:
Chromium(III) chloride forms many compounds with ammonia. To find the formula of one of these compounds, you titrate the NH3 in the compound with standardized acid. 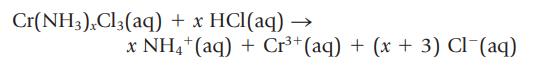
Assume that 24.26 mL of 1.500 M HCl is used to titrate 1.580 g of Cr(NH3)xCl3. What is the value of x?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





