(a) Write a balanced equation for the reaction of Al and H 2 O() to produce H...
Question:
(a) Write a balanced equation for the reaction of Al and H2O(ℓ) to produce H2 and Al2O3.
(a) Using thermodynamic data in Appendix L, calculate ΔrH°, ΔrS°, and ΔrG° for this reaction. Do these data indicate that the reaction should favor the products at equilibrium?
(b) Why is aluminum metal unaffected by water?
Data given in Appendix L
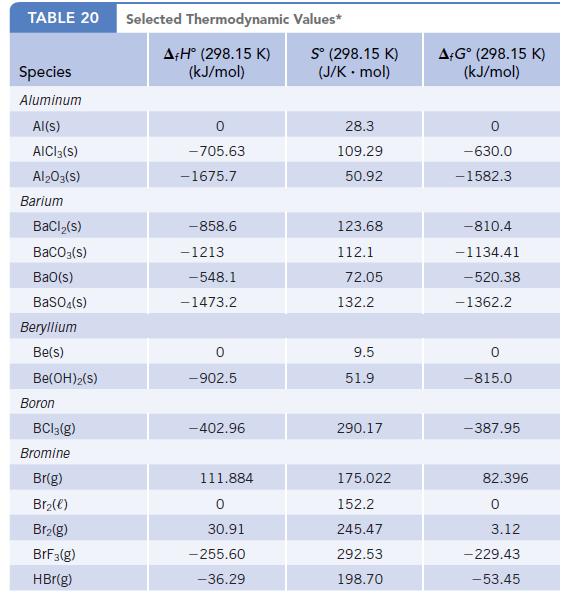
Transcribed Image Text:
TABLE 20 Species Aluminum Al(s) AICI 3(S) Al2O3(S) Barium BaCl₂(s) BaCO3(s) BaO(s) BaSO4(s) Beryllium Be(s) Be(OH)2(S) Boron BC13(g) Bromine Br(g) Br₂(e) Br₂(g) BrF3(g) HBr(g) Selected Thermodynamic A+Hº (298.15 K) (kJ/mol) 0 -705.63 -1675.7 -858.6 -1213 -548.1 -1473.2 -902.5 -402.96 111.884 0 30.91 -255.60 -36.29 Values* Sº (298.15 K) (J/K . mol) 28.3 109.29 50.92 123.68 112.1 72.05 132.2 9.5 51.9 290.17 175.022 152.2 245.47 292.53 198.70 A+Gᵒ (298.15 K) (kJ/mol) -630.0 -1582.3 -810.4 -1134.41 -520.38 -1362.2 0 -815.0 -387.95 82.396 0 3.12 - 229.43 -53.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Balanced Equation 2Als 6HOl 2AlOH3 s 3H2g Thermodynamic Data You would need specific ...View the full answer

Answered By

Arshad Ahmad
Well, I am really new to tutoring but I truly believe a good student can be a better teacher. I have always been a topper at school. I passed my Chartered Accountancy at a very young age of 23, a rare feat for most of the students. I am really dedicated to whatever work I do and I am very strict regarding deadlines. i am always committed and dedicated to whatever work allotted to me and I make sure it is completed well within deadline and also I try to give my best in whatever I do. Hope we will have a good time studying together.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The boron trihalides (except BF 3 ) hydrolyze completely to boric acid and the acid HX. (a) Write a balanced equation for the reaction of BCl 3 with water. (b) Calculate r H for the hydrolysis of...
-
The dissolved oxygen present in any highly pressurized, hightemperature steam boiler can be extremely corrosive to its metal parts. Hydrazine, which is completely miscible with water, can be added to...
-
Write a balanced equation for the reaction of -(1)-galactose (use either an acyclic or a cyclic structure, whichever seems more appropriate) with each of the following: a. Hydroxylamine (to form an...
-
You are given the following information concerning Parrothead Enterprises: Calculate the company?s WACC. Debt: 13,000 6.4 percent coupon bonds outstanding, with 15 years to maturity and a quoted...
-
Trial procedures are formal and involve a lot of people, whereas discovery procedures are relatively informal and involve relatively few people. Consequently, discovering a fact before trial is...
-
In problem, identify which of the graphs could be the graph of a polynomial function. For those that could, list the real zeros and state the least degree the polynomial can have. For those that...
-
Find the probability density function of the random variable \(Z\) in terms of the known density function \(p_{U}(u)\) when (a) \(z=a u+b\), where \(a\) and \(b\) are real constants. (b)...
-
1. What type of franchise was Del Reys La Grande Enchilada restaurant? 2. If Del Rey operates the restaurant as a sole proprietorship, then who bears the loss for the damaged kitchen? Explain. 3....
-
On December 31, Padre acquires Sol's outstanding stock by paying $154,000 in cash and issuing 16,700 shares of its own common stock with a fair value of $40 per share. Padre paid legal and accounting...
-
Write balanced equations for the reactions of aluminum with HCl(aq), Cl 2 , and O 2 .
-
Diborane can be prepared by the reaction of NaBH 4 and I 2 . Which substance is oxidized, and which is reduced?
-
Consider an open loop system having a transfer function G(s) = (s + 4)1 and a sampler at the input. Find its output c(t) by the conventional z-transform analysis, for a unit step input u(t) and also...
-
Amy Jones was physically and mentally abused by her husband, Mark, for the last 10 years of their marriage. Amy routinely ended up in the hospital as a result of Marks terrible beatings. One evening...
-
Jodie Matthews is the lead prosecutor on a murder trial that is currently in the process of jury selection. Juror #2, Lane Smith, claims that he was a college roommate of the defense attorney. Juror...
-
James Garfalo was arrested for selling 10 grams of cocaine to an undercover police officer. At his jury trial in the state trial court, James was convicted and sentenced to two years in prison. James...
-
Assume the maximum pressure ratio. Determine the efficiency and work ratio of the cycle in P17.1. \([0.75,0\). P17.1. A gas turbine engine operates between minimum temperature \(T_{1}\) and maximum...
-
On 1 December 20x0, the board of Progressive Corporation approved a share option plan for selected employees. Up to 5,000,000 ordinary shares could be issued under this option plan. The terms of the...
-
As of December 31, 2008, Delta Air Lines had the following account listed as a current liability on its balance sheet: Accrued salaries and related benefits........$1,367,000,000 The related benefits...
-
KD Insurance Company specializes in term life insurance contracts. Cash collection experience shows that 20 percent of billed premiums are collected in the month before they are due, 60 percent are...
-
Tom has two ways he could drive home from work. He could take Highway 99 for 45 miles with a speed limit of 65 mi/h, or he could take Interstate 5, which would take him a bit out of the way at 57...
-
According to Newtons third law, for every force there is always a reaction force of equal magnitude and opposite direction. In each of the examples below, identify an actionreaction pair of forces....
-
A cannon is fired horizontally from a platform (Fig. P2.49). The platform rests on a flat, icy, friction less surface. Just after the shell is fired and while it is moving through the barrel of the...
-
When you 'pop' popcorn, you are essentially conducting an experiment using Gay- Lussac's Law (there are a couple of other things going on here, too, but let's just focus on the gas laws). There is a...
-
Find the velocity, acceleration, and speed of a particle with position function r(t) = (6t sin(t), 6t cos(t), -8t) v(t) = a(t) = = |v(t)| =
-
STP PROCESS What's the overall concept of STP? What is it? Why would an organization need to undertake this strategy? What are the benefits? How does it relate to other parts of marketing? How would...

Study smarter with the SolutionInn App


