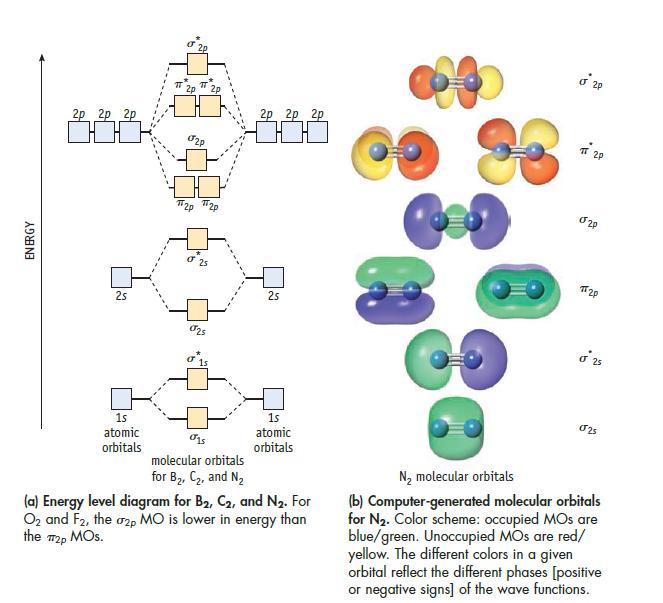
Assume the energy level diagram shown in Figure 9.16 can be applied to the heteronuclear molecule ClO.
Question:
Assume the energy level diagram shown in Figure 9.16 can be applied to the heteronuclear molecule ClO.
(a) Write the electron configuration for chlorine monoxide, ClO.
(b) What is the highest-energy, occupied molecular orbital (the HOMO)?
(c) Is the molecule diamagnetic or paramagnetic?
(d) What is the net number of σ and π bonds? What is the ClO bond order?
Data given in Figure 9.16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





