Boron forms a series of compounds with hydrogen, all with the general formula B x H y
Question:
Boron forms a series of compounds with hydrogen, all with the general formula BxHy.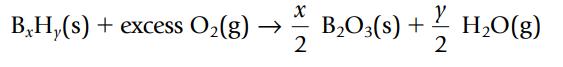
If 0.148 g of one of these compounds gives 0.422 g of B2O3 when burned in excess O2, what is its empirical formula?
Transcribed Image Text:
X B Hy(s) + excess O₂(g) → 2 Y B₂O3(s) + s) + 2/1/201 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To determine the empirical formula of the compound you need to find the ratio of the moles of boron ...View the full answer

Answered By

Robert Mwendwa Nzinga
I am a professional accountant with diverse skills in different fields. I am a great academic writer and article writer. I also possess skills in website development and app development. I have over the years amassed skills in project writing, business planning, human resource administration and tutoring in all business related courses.
4.90+
187+ Reviews
378+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Silicon and hydrogen form a series of compounds with the general formula Si x H y . To find the formula of one of them, a 6.22-g sample of the compound is burned in oxygen. All of the Si is converted...
-
Fluorine is so reactive that it forms compounds with materials inert to other treatments. (a) When 0.327 g of platinum is heated in fluorine, 0.519 g of dark red, volatile solid forms. What is its...
-
What makes the water move in a drip coffee brewer? The electrical pump pushes the water past the check valve and the heater up to the sprayhead The heater creates bubbles of steam, which expand and...
-
Given the following marginal utility schedule for good X and good Y for an individual A, given that the price of X and the price of Y are both $10, and that the individual spends all his income of...
-
Explain the importance of respect for people in JIT.
-
What is the quirk in the tax code that makes a levered firm more valuable than an otherwise identical unlevered firm?
-
Two sources, one of strength \(m\) and the other with strength \(3 m\), are located on the \(x\) axis as shown in Fig. P6.43. Determine the location of the stagnation point in the flow produced by...
-
Braxton Technologies, Inc., constructed a conveyor for A&G Warehousers that was completed and ready for use on January 1, 2011. A&G paid for the conveyor by issuing a $100,000, four-year note that...
-
An iron block with a mass of 45 000 g rests on an incline plane of 16 to the horizontal. Take the coefficient friction as 0,25. Calculate the following: 1.1 the weight component perpendicular to the...
-
Saccharin, an artificial sweetener, has the formula C 7 H 5 NO 3 S. Suppose you have a sample of a saccharin-containing sweetener with a mass of 0.2140 g. After decomposition to free the sulfur and...
-
Iodine is made by the following reaction (a) Name the two reactants. (b) If you wish to prepare 1.00 kg of I 2 , what masses of NaIO 3 and NaHSO 3 are required? (c) What is the theoretical yield of I...
-
Gary and Christina Worden own the Mainstreet Inn, a bed and breakfast, in Parkville, Missouri. A stone wall ran the length of the property behind the inn. A driveway and walkway along the wall were...
-
Explain why supervisors and non-HR managers are expected to be familiar with the basics of HRM. List five examples of the types of HR responsibilities supervisors are expected to perform.
-
How does regulation and legal standards apply to HIM in Veterinary Medical Records or hospital different from a human hospital? What would you do if you were an HIM Director or Manager...
-
A company has a semiannual coupon bond issue that has a coupon rate of 10%, a par value of $1,000, a 20 years maturity, and a current price of $1,319.42. Determine the bond's YTC if the bond is...
-
How many natural modes have five pendulums that are attached with a spring when they swing in any direction? Discuss.
-
The price of oil fell sharply in 1986 and again in 1998. A. Show the impact of such a change in both the aggregate-demand/aggregate-supply diagram and the Phillips-curve diagram. What happens to...
-
When disposing of an available-for-sale investment, where is the gain or loss on disposal reported in the financial statements?
-
1. True or False. Pitfalls to consider in a statistical test include nonrandom samples, small sample size, and lack of causal links. 2. Because 25 percent of the students in my morning statistics...
-
Identify the starting materials needed to make each of the following acetals: (a) (b) (c) OEt
-
Using ethanol as your only source of carbon atoms, design a synthesis for the following compound:
-
Propose an efficient synthesis for each of the following transformations: (a) (b)
-
Create system documentation to the given scenario including clear stepwise information and include the relevant diagrams. The Fire Department in Newtown has been restructured and is shortly opening a...
-
What is a primitive polynomial? Let's assume, you need to generate unique bit sequences of 14 periods. How many bits do you need to design the LFSR? List at least two feedback functions for your LFSR.
-
July 2021: The telco market in Singapore is intensely competitive. With the entrance of new digital only operators, the full-service incumbents are feeling the pressure. With a falling average...

Study smarter with the SolutionInn App


