Copper metal can be prepared by roasting copper ore, which can contain cuprite (Cu 2 S) and
Question:
Copper metal can be prepared by roasting copper ore, which can contain cuprite (Cu2S) and copper(II) sulfide.
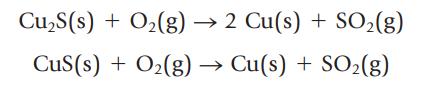 Suppose an ore sample contains 11.0% impurity in addition to a mixture of CuS and Cu2S. Heating 100.0 g of the mixture produces 75.4 g of copper metal with a purity of 89.5%. What is the weight percent of CuS in the ore? The weight percent of Cu2S?
Suppose an ore sample contains 11.0% impurity in addition to a mixture of CuS and Cu2S. Heating 100.0 g of the mixture produces 75.4 g of copper metal with a purity of 89.5%. What is the weight percent of CuS in the ore? The weight percent of Cu2S?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





