Dihydroxyacetone is a component of quick tanning lotions. (It reacts with the amino acids in the upper
Question:
Dihydroxyacetone is a component of quick tanning lotions. (It reacts with the amino acids in the upper layer of skin and colors them brown in a reaction similar to that occurring when food is browned as it cooks.)
(a) Use bond dissociation enthalpies to estimate the enthalpy change for the following reaction. Is the reaction exothermic or endothermic?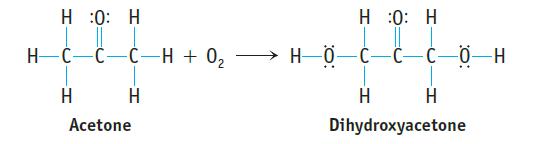
(b) Are dihydroxyacetone and acetone polar molecules?
(c) A proton (H+) can be removed from a molecule of dihydroxyacetone with strong bases (which is in part what happens in the tanning reaction). Which H atoms are the most positive in dihydroxyacetone?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





