Ethylenediamine, H 2 NCH 2 CH 2 NH 2 , can interact with water in two steps,
Question:
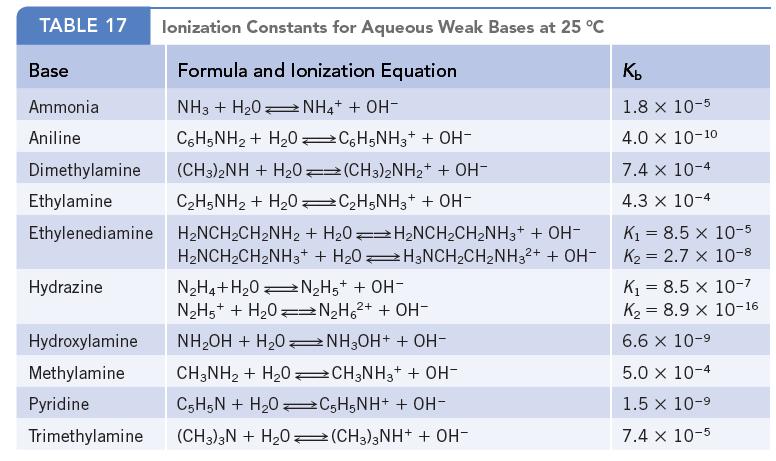
Ethylenediamine, H2NCH2CH2NH2, can interact with water in two steps, forming OH− in each step (Appendix I). If you have a 0.15 M aqueous solution of the amine, calculate the concentrations of [H3NCH2CH2NH3]2+ and OH−.
Data given in Appendix I
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





