Hydrazine, N 2 H 4 , can interact with water in two steps. (a) What is the
Question:
Hydrazine, N2H4, can interact with water in two steps.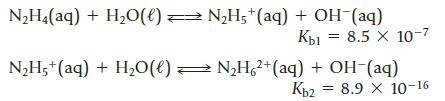
(a) What is the concentration of OH−, N2H5+, and N2H62+ in a 0.010 M aqueous solution of hydrazine?
(b) What is the pH of the 0.010 M solution of hydrazine?
Transcribed Image Text:
N2H4(aq) + H2O(0) = N2H5+(aq) + OH (aq) Кы 8.5 х 10-7 N2H5+(aq) + H2O(l) — N2H 2+(aq) + OH-(aq) Ког = 8.9 X 10-16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To solve this problem well first calculate the concentrations of OH N2H5 and N2H62 in a 0010 M aqueo...View the full answer

Answered By

SUMAN DINDA
I LIKE TO TEACH STUDENTS. SO, I START MYSELF AS A PRIVATE TUTOR. I TEACH STUDENTS OF DIFFERENT CLASSES. I HAVE ALSO DONE BACHELOR OF EDUCATION DEGREE(B.ED). DURING THIS COURSE I HAD TO TEACH IN A SCHOOL. SO I HAVE A GOOD EXPERIENCE IN TEACHING.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Ethylenediamine, H 2 NCH 2 CH 2 NH 2 , can interact with water in two steps, forming OH in each step (Appendix I). If you have a 0.15 M aqueous solution of the amine, calculate the concentrations of...
-
Two 0.10-mol samples of the hypothetical monoprotic acids HA(aq) and HB(aq) are used to prepare 1.0-L stock solutions of each acid. a. Write the chemical reactions for these acids in water. What are...
-
Information is one of the most important assets of any business. Businesses must ensure information accuracy, completeness, consistency, timeliness, and uniqueness. In addition, business must have a...
-
Two independent situations follow: 1. Ready Car Rental leased a car to Culver Company for three months. Terms of the lease agreement call for monthly payments of $885, beginning on May 21, 2024....
-
Explain the difference between a firms corporate strategy and business strategy. Why do firms need to look at both aspects?
-
What is the difference between social responsibility and managerial ethics?
-
Describe the two-plane balancing procedure.
-
Billabong Tech uses the internal rate of return (IRR) to select projects. Calculate the IRR for each of the following projects and recommend the best project based on this measure. Project T-Shirt...
-
Muthuphone Mobiles Inc. produces mobile phones in a factory in Dhaka, Bangladesh to satisfy some local demands. Output is measured in a number of units. Materials are added at the beginning of the...
-
Suppose a tank initially contains H 2 S at a pressure of 10.00 atm and a temperature of 800 K. When the reaction has come to equilibrium, the partial pressure of S 2 vapor is 0.020 atm. Calculate K p...
-
Ascorbic acid (vitamin C, C 6 H 8 O 6 ) is a diprotic acid (K al = 6.8 10 5 and K a2 = 2.7 10 12 ). What is the pH of a solution that contains 5.0 mg of acid per milliliter of solution? HO H H HO H...
-
Penron Limited (PL) is in the energy business of buying and selling gas and oil and related derivatives. It is a public company whose shares are widely held. It recently underwent a tremendous...
-
On March 1, 2019, Wanda Sykes drove her 2019 SUV through Bon Temps' main street. Wanda's phone rang at an intersection. She dropped it on the passenger side as she got in. Wanda's two-year-old twins,...
-
Chatman has pled guilty to possession of controlled substances and is pending sentencing. His attorney has told him that the judge usually orders everyone drug tested at sentencing and the result...
-
Outline the constitutional amendments that form the basis of the criminal justice system and explain the right afforded to the individual by that amendment. 2. Explain the US Supreme Court's role in...
-
The Court of Appeal case between Chan Sze Ying and the Management Corporation Strata Title Plan No 2948. Lee Chuen T'ng intervened in the case. The case involves an Originating Summons related to...
-
Judge Johnny is a state judge and sworn to uphold the laws of the state, Judge Johnny also has a moral reservation about the state's use of the death penalty as a punishment. He impanels a jury,...
-
Why is the reinvestment of interest income so important to bond investors?
-
After Theorem 1.5 we note that multiplying a row by 0 is not allowed because that could change a solution set. Give an example of a system with solution set S0 where after multiplying a row by 0 the...
-
a. The viscosity of Cl 2 at 293 K and 1atm is 132 P. Determine the collisional cross section of this molecule based on the viscosity. b. Given your answer in part (a), estimate the thermal...
-
a. The viscosity of O 2 at 293 K and 1atm is 204 P. What is the expected flow rate through a tube having a radius of 2.00 mm, length of 10.0 cm, input pressure of 765 Torr, output pressure of 760....
-
The Reynolds number (Re) is defined as Re = p(v x )d/, where and are the fluid density and viscosity, respectively; d is the diameter of the tube in which the fluid is flowing; and (v x )is the...
-
To walk his puppy on a sled, a man pulls it at an angle of 30. If the combined mass of the puppy and the sled is 31 kg. What is the force applied if the acceleration produced is 1.9 m/s? Answer: N 30...
-
Consider the following system of linear equations: 1+2x2+3x3 + 4x4 = 0, 4x1+5x2+6x3+7x4 = 0, and 6x1+7x2+8x3+9x4 = 0. (a) (12 pts) Find a basis for the column space of the coefficient matrix of this...
-
As countries recover from COVID-19, multinational companies' success and competitiveness will be determined by their ability to manage complexity efficiently and effectively in uncertain times:...

Study smarter with the SolutionInn App


