For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for
Question:
For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for the overall reaction.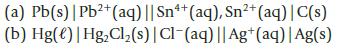
Transcribed Image Text:
(a) Pb(s) | Pb²+ (aq) || Sn4+ (aq), Sn²+ (aq) | C(s) (b) Hg()| Hg₂Cl₂ (s) | Cl-(aq) || Ag+ (aq) | Ag(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a Oxidation HalfReaction Pbs Pb2aq 2e Reduction Hal...View the full answer

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for the overall reaction. (a) Cu(s) | Cu+ (aq) || Fe+ (aq), Fe+ (aq) | Pt(s) (b)...
-
Each of the following equations describes a reaction of a compound called methyl formate. To what class of compounds does methyl formate belong? Which reactions require a reducing agent? Which...
-
Write balanced equations for each of the following reactions (some of these are analogous to reactions shown in the chapter). (a) Aluminum metal reacts with acids to form hydrogen gas. (b) Steam...
-
In Problems 65-72, summarize all pertinent information obtained by applying the graphing strategy, and sketch the graph of y=f(x). 66. 68. 70. 72. x2x6 f(x) 2x 1x +14 f(x)-_-x2-4 x3-5x2-6x 3x +2...
-
Prepare an income statement through gross profit for Brewster Company using the variance data in Practice Exercises 23-1A, 23-2A, 23-3A, and 23-4A. Assume Brewster sold 1,500 units at $80 per unit.
-
Consider a small open economy with a tradable goods sector and a nontradable goods sector. Only tradable goods can be traded internationally; nontradable goods have to be consumed domestically. The...
-
What is a linguistic variable? Give 10 examples of linguistic variables that you might use to describe a building.
-
Hart Lumber is considering the purchase of a paper company. Purchasing the company would require an initial investment of $300 million. Hart estimates that the paper company would provide net cash...
-
Chattanooga Co. has collected the following per unit data: Direct labor $5 Variable selling and admin. $8 Direct materials 7 Fixed overhead $6 Variable overhead 6 Fixed selling and admin. $7 with a...
-
Use cell notation to depict an electrochemical cell based upon the following reaction that is product-favored at equilibrium. Cu(s) + Cl(g) 2 Cl(aq) + Cu+ (aq)
-
Once you have separated the three salts in Study Question 111 into three test tubes, you now need to confirm their presence. (a) For Pb 2+ ion, one way to do this is to treat a precipitate of PbCl 2...
-
A metric space X is connected if and only if X and are the only sets that are both open and closed.
-
Write down the logic function implemented by the CMOS circuit of Figure 4.28(b). Data in Figure 4.28(b). T PFETS [ (b) X
-
The scatterplot shows the acceptance rate and selectivity index for a sample of medical schools. The acceptance rate is the percentage of applicants who were accepted into the medical school. The...
-
Skechers U.S.A., Inc., is a performance footwear company headquartered in Manhattan Beach, California. The sales revenue for Skechers over a four-year period are as follows: a. Are these...
-
Compute the maximum and minimum rise/fall times of the gates shown in Figure 5.18(b). Assume that only one input toggles at a time and that the gates drive an output of 4C inv . You may leave your...
-
Repeat Exercise 23.11 with the double buffer of Figure 23.11 and the VHDL of Figure 23.12. The fail signal should propagate by only one stage per cycle (like the ready signal). Data in Exercise 23.11...
-
1. Using the data in E, prepare a cost of production summary for the month of December for Finishing. In E, Micro Motors Inc. has two production departments. Finishing had 2,000 units in process at...
-
Prove that if Σ an is absolutely convergent, then a. an
-
A bullet of mass 10 g leaves the barrel of a rifle at 300 m/s. Assuming the force on the bullet is constant while it is in the barrel, find the magnitude of this force.
-
What is the approximate speed at which the force of air drag on a car is equal to the weight of the car?
-
A mass with M = 102 kg is attached to the bottom of a block-and-tackle pulley system as depicted in Figure P3.65. How much tension force is needed to keep the mass at its current position? Figure...
-
Write a proof below for the following statement. Let n be an integer. Show that if n is a multiple of 3, then n must also be a multiple of 3. (Hint: One of the best methods is to make a proof by...
-
How does an operating system ensure compatibility with a wide range of hardware through the use of device drivers, and what are the challenges involved in writing robust and secure drivers ?
-
b) (3 pts) Suppose you want to create a larger ROM to store a set of 256 different 5x8 pixel characters, specified by an 8-bit number (e.g. the so-called ASCII character code), such as in the figure...

Study smarter with the SolutionInn App


