Hydrazine and 1,1-dimethylhydrazine both react spontaneously with O 2 and can be used as rocket fuels. The
Question:
Hydrazine and 1,1-dimethylhydrazine both react spontaneously with O2 and can be used as rocket fuels.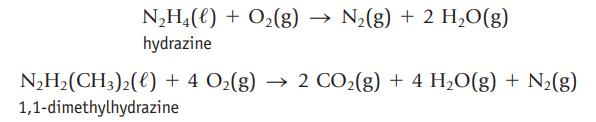
The molar enthalpy of formation of N2H4(ℓ) is +50.6 kJ/mol, and that of N2H2(CH3)2(ℓ) is +48.9 kJ/mol. Use these values, with other ∆fH° values, to decide whether the reaction of hydrazine or 1,1-dimethylhydrazine with oxygen provides more energy per gram
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





