(a) Calculate the enthalpy change, r H, for the formation of 1.00 mol of strontium carbonate...
Question:
(a) Calculate the enthalpy change, ∆rH°, for the formation of 1.00 mol of strontium carbonate (the material that gives the red color in fireworks) from its elements
![]()
The experimental information available is 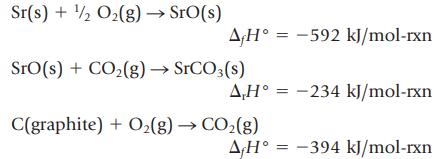
(b) Draw an energy level diagram relating the energy quantities in this problem.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





