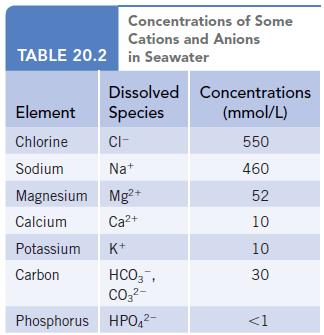
In any solution, there must be a balance between the positive and negative charges of the ions
Question:
In any solution, there must be a balance between the positive and negative charges of the ions that are present. Use the data in Table 20.2 to determine how close to a balance of charge is achieved for the ions in seawater. (The table lists only the major ions in seawater, and the balance between positive and negative charge will only be approximate.)
Data given in Table 20.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





