In some laboratory analyses, the preferred technique is to dissolve a sample in an excess of acid
Question:
In some laboratory analyses, the preferred technique is to dissolve a sample in an excess of acid or base and then “back-titrate” the unreacted acid or base with a standard base or acid. To assess the purity of a sample of (NH4)2SO4 you dissolve a 0.475-g sample of impure (NH4)2SO4 in aqueous KOH.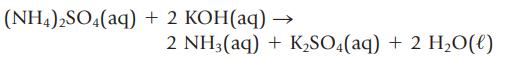
The NH3 liberated in the reaction is distilled from the solution into a flask containing 50.0 mL of 0.100 M HCl. The ammonia reacts with the acid to produce NH4Cl, but not all of the HCl is used in this reaction. The amount of excess acid is determined by titrating the solution with standardized NaOH. This titration consumes 11.1 mL of 0.121 M NaOH. What is the weight percent of (NH4)2SO4 in the 0.475-g sample?
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





