In the gas phase, acetic acid exists as an equilibrium of monomer and dimer molecules. (The dimer
Question:
In the gas phase, acetic acid exists as an equilibrium of monomer and dimer molecules. (The dimer consists of two molecules linked through hydrogen bonds.)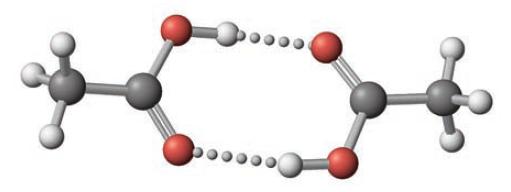
The equilibrium constant, Kc, at 25°C for the monomer–dimer equilibrium![]()
has been determined to be 3.2 × 104. Assume that acetic acid is present initially at a concentration of 5.4 × 10−4 mol/L at 25°C and that no dimer is present initially.
(a) What percentage of the acetic acid is converted to dimer?
(b) As the temperature increases, in which direction does the equilibrium shift? (Recall that hydrogen-bond formation is an exothermic process.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





